Luis Alberto Saraiva SantosI; Anderson BenícioII; Ewaldo de Mattos JúniorIII; Luiz Alberto BenvenuttiIV; Idágene Aparecida CestariV; Noedir Antonio Groppo StolfVI; Luiz Felipe Pinho MoreiraVII
DOI: 10.5935/1678-9741.20120097
ABSTRACT
OBJECTIVE: Right ventricular (RV) failure during left ventricular assist device (LVAD) support can result in severe hemodynamic compromise with high mortality. This study investigated the acute effects of cavo-pulmonary anastomosis on LVAD performance and RV myocardial compromise in comparison with biventricular circulatory support, in a model of biventricular failure.
METHODS: LVAD support was performed by centrifugal pump in 21 pigs with severe biventricular failure obtained by FV induction. Animals were randomized to be submitted to cavo-pulmonary anastomosis, to biventricular circulatory support or to control group. They were maintained under circulatory support and hemodynamic monitoring for 3h. Venous lactate and cytokines serum levels were also obtained. Endocardium samples were analyzed by electronic microscopy.
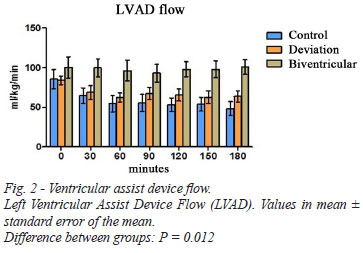
RESULTS: FV maintenance was responsible for acute LVAD impairment after 180 min in the control group. cavo-pulmonary anastomosis resulted in non-significant improvement of LVAD pump flow in relation to control group (+55±14 ml/kg/min, P=0.072), while animals under biventricular support maintained higher LVAD flow (+93±17 ml/kg/min, P=0.012). Mean arterial pressure remained constant only in biventricular group (P<0.001), which also presented decrease of right atrial and ventricular pressures. Similar increases in lactate and cytokines levels were observed in the three groups. Ultra-structural analysis documented low levels of myocardial swelling in the biventricular group (P=0.017).
CONCLUSION: The concomitant use of cavo-pulmonary anastomosis during LVAD support in a pig model of severe biventricular failure resulted in non-significant improvement of hemodynamic performance and it did not effectively replace the use of biventricular support.
RESUMO
OBJETIVO: Este estudo avaliou o desempenho hemodinâmico e as alterações miocárdicas decorrentes do emprego de dispositivos de assistência ventricular esquerda (DAVE), associado ou não à descompressão do ventrículo direito por meio de derivação cavo-pulmonar, sendo esses achados comparados ao emprego de assistência circulatória biventricular.
MÉTODOS: Vinte e um suínos foram submetidos à indução de insuficiência cardíaca através de fibrilação ventricular, sendo a atividade circulatória mantida por DAVE durante 180 minutos. No grupo controle, foi apenas implantado o DAVE. No grupo derivação, além do DAVE foi realizada cirurgia de derivação cavo-pulmonar. No grupo biventricular, foi instituída assistência biventricular. Foram monitoradas as pressões intracavitárias por 3 horas de assistência e amostras do endocárdio dos dois ventrículos foram coletadas e analisadas à microscopia óptica e eletrônica.
RESULTADOS: O lactato sérico foi significativamente menor no grupo biventricular (P=0,014). A diferença observada entre o fluxo do DAVE nos grupos derivação e controle (+55±14 ml/kg/min, P=0,072) não foi significativa, enquanto que o fluxo no grupo biventricular foi significativamente maior (+93±17 ml/kg/min, P=0,012) e se manteve estável durante o experimento. A pressão arterial média (PAM) se manteve constante apenas no grupo biventricular (P<0,001), que também apresentou diminuição significativa das pressões em câmaras direitas. Na análise ultraestrutural, notou-se menor presença edema miocárdico no ventrículo direito no grupo biventricular (P=0,017).
CONCLUSÃO: Os resultados apresentados demonstram que o desempenho hemodinâmico da assistência ventricular esquerda associada à derivação cavo-pulmonar, neste modelo experimental, não foi superior ao observado com a assistência de ventrículo esquerdo isolada e não substituiu a assistência biventricular de maneira efetiva.
LA: Left atrium
LVAD: Left ventricular assist devices
VF: Ventricular fibrillation
EM: Transmission electron microscopy
OM: Optical microscopy
LAP: Left atrial pressure
MAP: Mean arterial pressure
PAP: Pulmonary artery pressure
PTFE: Expanded polytetrafluoroethylene
CVP: Central venous pressure
RVP: Right ventricular pressure
RV: Right ventricle
LV: Left ventricle
INTRODUCTION
Heart failure has been associated with poor prognosis and high morbidity and, despite optimal medical therapy, mortality remains unacceptably high, which justifies the search for alternative treatments. In this context, we can find mechanical circulatory support devices, which provide for this group of patients better quality and life expectancy [1-3].
One of the main complications of the implantation of left ventricular assist devices (LVAD) alone is the circulatory dysfunction of the right ventricle (RV), which has an incidence varying between 13% and 44% [1,3,4]. Although controversial, most authors report that the institution of biventricular assistance should be performed as early as possible if the RV failure is hemodynamically important [4,5]. But with double of cannulation and pumps "sites", the adverse effects of this type of circulatory support increase[6].
Another alternative that has been suggested recently to acute right ventricular failure is the use of its surgical volume decompression volume through the cavopulmonary shunt [7,8]. The effectiveness of this procedure has been proven previously in experimental studies [9] and there is one report in the literature of patients who obtained clinical success of weaning from RV circulatory assist device, facilitated by the construction of cavopulmonary shunt when in use of biventricular assist device [8].
The aim of this study was to assess the hemodynamic performance and myocardial changes in the employment of LVAD, with or without RV decompression through cavopulmonary anastomosis in an experimental model of acute biventricular dysfunction in pigs and to compare these effects to those seen with the use of biventricular circulatory assistance. The influence of the types of circulatory assistance imposed on inflammatory response and tissue perfusion of animals was also assessed.
METHODS
Twenty-one pigs, weighing between 25-35 kg, underwent induction of acute biventricular failure obtained from the onset of the rhythm of ventricular fibrillation (VF) by direct contact of a 12 volt electric charge battery with the anterior heart wall. The circulatory activity was maintained by the institution of LVAD. This study was approved by the Research Ethics Committee of the institution under protocol (SDC-1649/00/10) and all animals underwent surgery according to the rules set in "Manual on the Care and Use of Laboratory Animals" and "European Convention on Animal Care".
Protocol of animal experiments
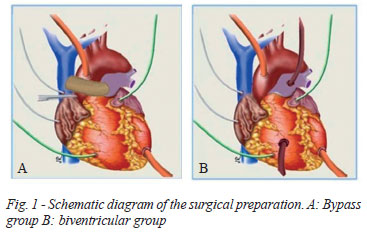
The animals were randomly divided into three groups of seven pigs, called control group, bypass group and biventricular group. The surgical preparation was similar in the three groups. In the control group, LVAD was installed with centrifugal pump (Biopump, Medtronic, Inc.) with aortic cannulation, with angled 12 Fr wireframe arterial cannula (Medtronic, Inc.) and the tip of the left ventricle (LV), with single stage wireframe 24 Fr cannula (Medtronic, Inc.). In bypass group (Figure 1A), in addition to biopump on the left side of the heart was performed cavopulmonary anastomosis between the superior vena cava and the pulmonary artery using a non-wired Nº 16 expanded polytetrafluoroethylene (PTFE) tube, which was made before the circulatory assistance. In the biventricular group (Figure 1B), in addition to the left circulatory support, biopump has also been installed on the right side of the heart, through cannulation of the pulmonary artery and right atrial appendage, with tubes similar to those previously described.
Anesthetic and surgical preparation
The animals underwent general anesthesia with ketamine (30 mg/kg im), midazolam (0.2 mg/kg, intravenously) and fentanyl (0.005 mg/kg, intravenously). Additional doses of fentanyl were administered as needed. Intubation using 6.5 Fr cannula was performed and then a ventilator was introduced (Harvard ventilator 708, South Natik, MA, USA). Before the surgery, electrodes were placed on the animal, for continuous electrocardiogram recording.
A venous line (right femoral vein) was accessed for collection of serum samples, and if required, additional volume infused. For fluid infusion, a maximum of 100 ml/kg was established in order to keep the central venous pressure (CVP) at normal values (10-14 mmHg). The temperature was obtained through a sensor inserted into the rectum, urinary output was measured by catheterization through cystostomy. Vasoactive drugs were not used in this protocol. An arterial line (right femoral artery) was obtained for monitoring of mean arterial pressure (MAP) and blood gas assessment and the right internal jugular vein was dissected for CVP monitoring.
The exposure of the heart was obtained through a median sternotomy and, after opening the pericardial sac, purse string sutures were performed in the left atrium (LA) and RV and micromanometers were inserted (5F, Model PC-350, Millar Instruments, Inc. Houston, USA) for continuous monitoring of intracavitary pressures. All sutures were performed in purses using prolene 4.0 wire, except the tip of the left ventricle, which was performed using Mersilene 2.0 polifilamentar wire.
After completion of the monitoring and cannulation of the animals, FV was induced, which was maintained by itself because it is not reversed by means of electrical defibrillation. The flow was maintained as large as possible, taking as parameter the LA pressure, which had as its goal the value close to zero, thus connoting good blood return to the pulmonary territory and good LV drainage. In biventricular group, the begining of assistance at the right side of the heart was simultaneously to the left and in this flow was maintained at values lower than 20% than the left sides.
Hemodinamic assessment
The LV rhythm was maintained for 180 minutes and during this period were recorded MAP, CVP, RV pressure (PVD) and left atrial pressure (LAP) in 30-minute intervals until the end of the protocol.
Evaluation of tissue perfusion and inflammatory response
Changes in tissue perfusion and myocardial infarction were assessed before the procedure and every 30 minutes through collection of blood samples for analysis of blood gases, hematocrit, and lactate. Samples were collected for analysis of changes in the inflammatory response at the time of initial preparation and subsequently every 60 minutes during the period of circulatory support. Serologic blood tests of TNF α, interleukin-1β and interleukin-6 through specific antibody for pigs (Duo-Set, R & D Systems, Minneapolis, MN, USA) were performed.
Ultramicroscopic assessment
Changes of myocardial cells in the three groups were assessed by optical microscopy (OM) and transmission electron microscopy (EM). Samples were taken at the RV free wall, in the interventricular septum and the LV free wall, obtained at the end of the experiment by removing the heart. The examiner was blind to the groups, after the result/report of the pathologist in charge, groups were revealed and the final result of this data was then made.
The samples were assessed by optical microscopy, being noted that they could be grouped into three distinct patterns of progressive gravity, as follows:
• Grade 0/+: Myocardium preserved or presenting small and scattered foci of recent necrosis of cardiomyocytes, characterized by cytoplasmic hypereosinophilia, contraction bands and nuclear pyknosis present only in the subendocardial region which presents no interstitial hemorrhage.
• Grade ++: Multiple foci of recent necrosis of cardiomyocytes in the subendocardial region, encompassing groups of cells, characterized by cytoplasmic hypereosinophilia, contraction bands and nuclear pyknosis, with or without small foci, sparse, of necrosis in mid-layer wall. It is the usual the presence of foci of hemorrhage in the subendocardium.
• Grade +++: Multiple foci of recent necrosis of cardiomyocytes in subendocardial and mid-mural region encompassing clusters of cells, characterized by cytoplasmic hypereosinophilia, contraction bands and nuclear pyknosis. Foci of hemorrhage in the subendocardium and crisp interstitial edema.
Under EM, the presence or absence of cellular swelling and swollen mitochondria, electron-dense bodies and lysis of myofilaments was assessed, and the first two being considered lesions of mild to moderate intensity, and the last two, lesions of moderate to severe intensity. The cellular edema was characterized by the presence of one or more areas of clear separation of organelles by edema. The mitochondrial swelling was defined by the presence of irregular areas of vacuolization of the mitochondrial matrix, sometimes rupture of ridges present in several mitochondria. The electron-dense bodies were defined by the presence of multiple electron-dense corpuscles in the mitochondrial matrix and, ultimately, myofilaments lysis was characterized by the presence of multiple areas of dissolution of myofilaments sarcomeres.
To avoid the appearance of post-mortem lesions that might distort the analysis of the material after removal of the heart, it was set maximum time of 15 minutes between the removal of the organ and fixing the material in glutaraldehyde.
Statistical Analysis
The statistical analysis was performed using the Graphpad Prism 5.2 software. The data were statistically assessed according to the type of distribution of variables. Parametric variables were expressed as mean ± standard error of the mean and were assessed by the two-tailed test of variance with repeated measures on the factor "time", complemented by the Bonferroni t test. The variables of non-parametric distribution were expressed as medians and percentiles, and were assessed by profile analysis. The level of significance was set at 5%.
RESULTS
After the phase of protocol standardization, 21 full experiments were performed, seven in each group. The weight of the animals ranged from 25 to 32 kg, with a mean of 29 ± 3 kg, 30 ± 3 kg and 27 ± 4 kg in the control groups, bypass and biventricular, respectively. Comparable amount of fluid was administered in the three groups. The values of arterial blood gases, hematocrit and hemoglobin were similar between groups. Serum lactate was significantly lower in biventricular group (Table 1).
Hemodynamic performance assessment
The hemodynamic data are shown in Table 2. LVAD flows observed were similar in the control groups and cavopulmonary bypass, while flow in biventricular group was significantly higher throughout the experiment (Figure 2).
The study of the pressure behavior in the left circulation has shown the maintenance of normal levels of MAP throughout the period of circulatory assistance only in the biventricular group, while the other groups showed a progressive decrease of this parameter. This drop, however, was not statistically significant and shows satisfactory maintenance of levels by the end of the experiments in the three groups studied (Figure 3A). The LVP assessment demonstrated effective performance of left ventricular assist in all experiments (Figure 3B).
Regarding the behavior of the pressure in the right chambers, we observed the existence of lower values only in the biventricular group, being observed similar values of pressure in the superior vena cava and right ventricle of the animals in the control group and cavopulmonary bypass group (Figures 4A and 4B).
Inflammatory response assessment
The assessment results of the inflammatory response through serum cytokine dosage are presented as medians and percentiles. Although there was a trend to higher levels of TNFa with maintaining circulatory assistance for longer periods in the biventricular group, this difference was not significant from a statistical standpoint. There were also no significant differences between the groups in relation to the interleukins 6 and 1β values (Figure 5).
Assessment of changes in myocardial cells
Table 3 shows the changes under OM observed in the groups. There were no significant differences in the degree of damage of myocardial fibers in the LV and RV, while the biventricular group showed fewer changes considered more severe in the septum.
The results of the changes observed under EM are shown in Table 4. We can observe the occurrence of more cases with the presence of cellular edema and mitochondrial edema in the control group and cavopulmonary bypass group in relation to the biventricular group in the RV free wall. The increased presence of cellular edema in the interventricular septum also observed in the control group and cavopulmonary bypass group, but was not significant from a statistical standpoint.
DISCUSSION
The RV failure occurs in 13% to 44% of patients undergoing implantation of LVAD devices alone, being the major cause of postoperative mortality of this procedure [1]. The use of uni- or biventricular assistance is the key to therapeutic success in treating patients with heart failure [10,11]. However, it is difficult to predict who patients are at increased risk of developing RV failure, because there is no consensus on criteria for preoperative risk among these studies [10-12].
Cavopulmonary anastomosis seems to be able to increase the effective pulmonary flow because volumetrically decompresses the RV, restoring its geometric shape [7,13]. There are clinical [8] and experimental [7,9] suggestions in the literature that the partial exclusion of the RV is beneficial in the treatment of right failure.
Danton et al. [9] demonstrated in an experimental model of acute myocardial infarction in pigs, the effectiveness of cavopulmonary anastomosis in RV decompression volume. Succi et al. [7] succeeded in demonstrating an experimental model in dogs that cavopulmonary anastomosis is able to provide adequate support to the RV, when combined with mechanical assistance to LV in biventricular failure. However, despite hemodynamic improvement evidenced in this study, the authors received major criticism for certain technical aspects, as the local drainage of the left system, which was performed by LA, the short time in which the animal was kept in failure (90 minutes), and questions about the choice of the use of continuous flow pumps and non-pulsatile flow.
In the present study, when the cavopulmonary anastomosis associated to LAVD was compared to LVAD alone, unlike the previous study, there was no significant hemodynamic improvement with the use of the method as compared to control, both being lower than the biventricular circulatory assistance. Perhaps technical changes in the performance of the cavopulmonary anastomosis, as the anastomosis in the pulmonary artery trunk and the use of synthetic tube instead of autologous tissue, have influenced results.
Classic cavopulmonary anastomosis already clinically demonstrated efficacy in the presence of circulatory support in a situation in which it was helpful in the case of a patient with biventricular assist weaning with the right circulatory support facilitated by such surgery [8]. The option of making the cavopulmonary anastomosis was modified motivated by technical difficulties in performing the classic form. As a result we chose to use the synthetic tube (PTFE Nº 16), which, despite having adequate caliber, it is speculated that it has less complacency than the native tissue vena cava. Danton et al. [9] performed an experimental study of acute RV failure and obtained satisfactory decompression of this chamber, when performing cavopulmonary anastomosis using modified autologous inferior vena cava, demonstrating the effectiveness of the method to decompress the RV presenting acute failure.
Another factor to be considered is again the time, because even though the animals have been exposed in this study to VF twice as long compared to the protocol used previously in the study by Succi et al. [7], there was no significant hemodynamic deterioration during the period of observation, and so maybe cavopulmonary anastomosis decompression has not shown the expected hemodynamic efficacy.
It should also be highlighted the question, in the present study, the local chosen for left drainage was the LV, which is set as the default location for this type of assistance [14], perhaps contributing to the less pronounced hemodynamic failure observed in this model. The use of pigs in this experiment, instead of dogs, directly influenced the final outcome, because they are different species with different behavioral and physiological responses to trauma.
There are numerous causes of failure of Glenn' surgery, including variations in pulmonary artery pressure, and high pulmonary vascular resistance among the major causes. In this sense there are recommendations, not consensual, to perform such surgery only in cases where the pulmonary artery pressure (PAP) is less than 18 mmHg, and ideally less than 15 mmHg, with pulmonary vascular resistance less than 2.0 Wood units [ 13]. In the present study, we used young animals without previous diseases, a fact that makes it unlikely that they have some kind of native pulmonary vascular disease. Furthermore, PAP was measured prior in all animals, which was within normal limits.
Moreover, it is known that the biventricular assistance is effective in decompressing the dilated RV, when it enters into failure and there are suggestions in the literature that such behavior should be instituted as early as possible in order to avoid irreversible damage to target organs [14,15]. However, despite the observed hemodynamic improvement, it is known that this type of assistance, despite the technological advances offered by new devices, it still remains high incidence of complications [7,14,16]. To that end, numerous studies are listed in an attempt to elucidate the criteria that could predict which patients are at higher risk of developing RV failure after the LVAD institution.
In the experimental model presented here, the circulatory support was instituted by centrifugal pump. This system is widely available in a specialized environment, has low cost, is easy to handle and there are several reports of successful clinical use in the literature. However, there are guidelines that its use should not be extended, because this type of assistance mechanism exacerbates the inflammatory response over time [17]. This statement is controversial and some studies claim that the low flow resulting from circulatory collapse, common at the time of institution of this kind of assistance would be primarily responsible for this exacerbation, and so successfully demonstrated that, after normalization of tissue perfusion obtained with assistance, there is decrease in inflammatory cytokines [18]. This observation corroborates the findings of this protocol, which showed no significant increase in inflammatory cytokines studied thus assuming that there was adequate tissue perfusion during the experiment.
Regarding the assessment of myocardial cells, it has been demonstrated in experimental model of infarction in pigs that irreversible ultrastructural lesions begin in just 15 minutes and that in 30 minutes, mitochondrial destruction occurs, thus characterizing cell death [19]. Moreover, it is widely accepted that during acute myocardial infarction myocardial ischemia propagates from subendocardial region to epicardial region, a phenomenon called "Wavefront Phenomenon" [20]. It has also been shown the occurrence in dilated cardiomyopathy, early ultrastructural changes, characterized by degeneration of mitochondrial and myofibrillar lysis [21].
Based on these concepts, samples collected from the subendocardial region of the RV, LV and septum were assessed under OM and EM. Under OM and EM, we observed decrease in the occurrence of cellular and mitochondrial swelling in the RV in the biventricular group. This fact can be explained by adequate drainage provided with such assistance, which decreases the chance of endocardial injury by increase of intracavity voltage.
The outcomes demonstrated in this study are subject to several limitations. Again, the short observation period can be considered the major limitation. Moreover, technical problems such as the assistance provided by centrifugal pumps and cannulae used in the experiments that were not manufactured for such purpose, may be greatly influenced the quality of the circulatory assist provided in the model. It is woth emphasizin that these are closer to the reality experienced by most specialized centers in our country. Functional evaluation methods, such as echocardiography, have their effective in assessing cardiac function in pigs proven in the literature and would be useful in this protocol [22]. In addition to these methods, it has recently been demonstrated that pulsatile flow pumps are as effective as the continuous flow pumps in relation to hemodynamic performance, but with less activation of part of the inflammatory system [23]. Another factor of great importance was the direct measurement of PAP, through which it would be possible to calculate pulmonary vascular resistance, which may have influenced the proper functioning of the cavopulmonary shunt.
The results of this study demonstrated that RV volumetric decompression through the cavopulmonary anastomosis modified in acute biventricular failure, while using LVAD mechanisms alone, was not superior than that observed by the institution of biventricular assistance and therefore should not be used as routine in surgical practice. However, more studies are needed to define the use of biventricular assist as standard procedure in the presence of acute RV failure.
REFERENCES
1. Kukucka M, Stepanenko A, Potapov E, Krasbatsch T, Redlin M, Mladenow A, et al. Right-to-left ventricular end-diastolic diameter ratio and prediction of right ventricular failure with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2011;30(1):64-9. [MedLine]
2. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATH) Study Group. Long-term use of left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435-43. [MedLine]
3. Kaul TK, Fields BL. Postoperative acute refractory right ventricular failure: incidence, pathogenesis, management and prognosis. Cardiovasc Surg. 2000;8(1):1-9. [MedLine]
4. Chen JM, Levin HR, Rose EA, Addonizio LJ, Landry DW, Sistino JJ, et al. Experience with right ventricular assist devices for perioperative right-sided circulatory failure. Ann Thorac Surg. 1996;61(1):305-10.
5. Hetzer R, Portner PM. Discussion of univentricular versus biventricular support. Ann Thorac Surg. 1996;61:357-8.
6. Loforte A, Monica PL, Montalto A, Musumeci R. HeartWare third-generation implantable continuous flow pump as biventricular support: mid-term follow-up. Interact Cardiovasc Thorac Surg. 2011;12(3):458-60. [MedLine]
7. Succi GM, Moreira LF, Leirner AA, Silva RS, Stolf NA. Cavo-pulmonary anastomosis improve left ventricular assist device support in acute biventricular failure. Eur J Cardiothorac Surg. 2009;35(3):528-33. [MedLine]
8. Martin JP, Allen JG, Weiss ES, Vricella LA, Russel SD, Conte JV. Glenn shunt facilitated weaning of right ventricular mechanical support. Ann Thorac Surg. 2009;88(3):e16-7.
9. Danton MH, Byrne JG, Flores KQ, Hsin M, Martin JS, Laurence RG, et al. Modified Glenn connection for acutely ischemic right ventricular failure reverses secondary left ventricular dysfuction. J Thorac Cardiovasc Surg. 2001;122(1):80-91. [MedLine]
10. Farrar DJ, Hill JD, Pennington DG, McBride LR, Holman WL, Kormos RL. Preoperative and postoperative comparison of patients with univentricular and bivaentricular support with the thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113(1):202-9. [MedLine]
11. Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative toll for assessing the risk of ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51(22):2163-72. [MedLine]
12. Fitzpatrick JR 3rd, Frederick JR, Hsu VM, Kozin ED, O'Hara ML, Howell E, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008;27(12):1286-92. [MedLine]
13. Freedom RM, Nykanen D, Benson LN. The physiology of bidirectional cavo-pulmonary connection. Ann Thorac Surg. 1998;66(2):664-7. [MedLine]
14. Stone ME. Current status of mechanical circulatory assistance. Semin Cardiothorac Vasc Anesth. 2007;11(3):185-204. [MedLine]
15. Fitzpatrick JR 3rd, Frederick JR, Hiesinger W, Hsu VM, McCormick RC, Kozin ED, et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared to delayed conversion of left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg. 2009;173(4):971-7.
16. Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Bhama JK, Bermudez CA, et al. Early adverse events as predictors of 1-year mortality during mechanical circulatory support. J Heart Lung Transplant. 2010;29(9):981-8. [MedLine]
17. Pedemonte VO, Aránguiz Santander E, Torres HH, Merello NL, Vera PA, Díaz NR, et al. Asistecia ventricular derecha com bomba centrifuga. Rev Med Chile. 2008;136(3):359-66.
18. Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J. 1998;19(5):761-5. [MedLine]
19. Spinale FG, Schulte BA, Crawford FA. Demonstration of early ischemic injury in porcine right ventricular myocardium. Am J Pathol. 1989;134(3):693-704. [MedLine]
20. Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56(5):786-94. [MedLine]
21. Jindal N, Talwar KK, Chopra P. Ultraestructural and histological study of endomyocardial biopsies from patients of dilated cardiomyopathy: a comparative evaluation and their clinical correlation. Indian Heart J. 1994;46(6):329-34. [MedLine]
22. Korosoglou G, Hansen A, Bekeredjian R, Filusch A, Hardt S, Schellberg D, et al. Usefulness of myocardial parametric imaging to evaluate myocardial viability in experimental and clinical studies. Heart. 2006;92(3):350-6. [MedLine]
23. Loebe M, Koster A, Sänger S, Potapov EV, Kuppe H, Noon GP, et al. Inflammatory response after implantation of a left ventricular assist device: comparison between the axial flow MicroMed DeBakey VAD and pulsatile Novacor device. ASAIO J. 2001;47(3):272-4. [MedLine]
Support: Foundation for Research Support of the State of São Paulo (FAPESP).
Article receive on Tuesday, May 8, 2012
 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license