INTRODUCTION
Cryopreserved valvar homografts are being utilized for the correction of diverse valvar and complex congenital heart diseases with very satisfactory functional results. Because of its physiological hemodynamic performance, the almost zero risk of thromboembolism and the high resistance to infection, they are considered the first choice in grafts in differing clinical situations [1].
Although durability is greater than other substitute biological valves, the homografts are also subject to progressive degeneration, especially affecting children and young adults. For this reason, several authors have recommended Ross operation for aortic valve replacement in patients of this age range [2].
The mechanisms of calcification and degeneration in substitute biological valves are complex involving multiple factors and they are still not completely understood. Among these, the controversial "harmful action" of glutaraldehyde, the presence of cellular remains which may serve as an initial focus for calcification and the reduced flexibility of the collagen fibers induced by cross-links, create conditions that facilitate the calcification process [3]. Additionally, the antigenicity of the tissue induces the activation and adherence of macrophages, which, when stimulated, can acquire a phenotype of osteoblasts (calcium deposits) [4,5].
The implantation of valvar homografts triggers an immunological response in the recipient, with activation of the T-lymphocytes and production of antibodies of the HLA system against type I and II histocompatibility antigens, which are basically found in the fibroblasts and in the endothelial cells of these grafts [6-8]. Although Welters et al. [9] suggested that the intensity of this reaction increases the probability of graft dysfunction, the correlation between the immunological response and the late results did not confirmed this in different published works. Curiously, the best late results were obtained with homovital and cryopreserved homografts, exactly those that have the greatest immunological potential [1,2].
More recent researches aim at, by means of tissue engineering, developing valves that incorporate autologous cells capable of making repairs and remodeling of the extracellular matrix, thus presenting with regenerative and growth capabilities, with the potential of greater durability [10-18].
The Tissue Engineering Laboratory of PUC in Parana, Brazil together with the Humboldt University of Berlin developed a method of decellularizing valvar homografts and heterografts, maintaining the extracellular collagen matrix intact. Grafts treated like this would be inert and capable of being repopulated with autogenous cells after their implantation [10].
The objective of this study was to comparatively evaluate the behavior of cryopreserved decellularized valvar homografts and heterografts, implanted in the outflow tract of the right ventricle of juvenile sheep. Additionally, the study aims to report the preliminary clinical experience with the employment of decellularized valvar homografts in patients submitted to the Ross operation.
METHOD
Preparation of the cryopreserved homografts
Ovine pulmonary homografts were obtained from juvenile Suffolk sheep with ages ranging from 4 to 6 months.
After sterilization in a nutrient solution with antibiotics (RPMI solution with Frademicine 120µg/mL, Cefoxitin 240µg/mL, Vancomycin 50µg/mL and Polymyxin B 100µg/mL) for 48 hours the grafts were frozen at - 1 ºC / minute in a Planer Cryoseries III computerized freezer. After they were stored in liquid nitrogen vapor at -150 ºC until the time of implantation.
Preparation of the decellularized porcine heterografts
Porcine pulmonary heterografts were sterilized in a nutrient solution containing antibiotics (RPMI solution with Penicillin 100U/mL, Streptomycin 100 mg/mL and Anfotericin B 250 ng/mL) for at least seven days. Consequently, they were submitted to decellularization using the method patented by the Humboldt University of Berlin, that includes the use of deoxycholic acid and ethanol. They were then maintained in a physiologic saline solution until the time of implantation.
Operative technique
In all the animals, the criteria of the guidelines for animal care elaborated by the Research Ethics Committee of PUC-Parana were followed.
Eight animals with ages ranging from 3 to 6 months and weights of from 25 to 40 kg underwent surgery. Four animals were implanted with cryopreserved homografts (Group A) and the other four with decellularized heterografts (Group B).
The animals fasted for 48 to 72 hours before the operation and were pre-medicated with 0.5 mg/kg Diazepam and 0.4 mg/kg Butorphanol. Anesthesia was induced with 5 mg/kg Ketamine and 3.5% Halothane using a mask and it was maintained with 1.5% Halothane and 0.8 mg/kg Propofol. The sheep were ventilated on an Oxigel 1700 respirator to keep the pCO2 between 35 and 45 mmHg. During the operation, electrocardiographic trace, invasive arterial pressure monitoring, pulse oximetry and capnography control were performed as well as serial gasometric analysis.
The operations were made by left lateral thoracotomy in the 3rd and 4th intercostal spaces, with the pericardium opened vertically to access the heart. After systemic anticoagulation using 250 IU/kg, the cardiopulmonary bypass was initiated using cannulation of the descending thoracic artery and single venous cannulation of the right atrium. Braile Biomedica pediatric membrane oxygenators were utilized, with systemic flow of 2.5 L/min/m2 of body surface at normothermia.
The pulmonary artery branch was dissected and freed from the neighboring structures and cross-sectioned just above the valve. The native pulmonary valve was totally resected and subsequently, the grafts were implanted using running sutures of 4-0 polypropylene thread both proximally and distally.
After finishing the anastomoses, the cardiopulmonary bypass was discontinued without the heparin being reverted. Hemostasis was reviewed, the thorax was closed by layers but leaving a thoracic drain in the pleural cavity with continuous suction until the extubation of the animal.
Antibiotic prophylaxis was achieved using 3 mg/kg Cephtiofur and 3 mg/kg Gentamicin endovenously, starting 6 hours before the surgery and continuing to the 5th postoperative day. The animals were maintained under observation in an experimental farm of PUC-Parana until the moment of sacrifice and removal of the grafts.
In each group, two animals were sacrificed on the 90th postoperative day and the other two at 150 days of evolution.
Echocardiographic control
All the animals were submitted to control echocardiograms on the 30th and 90th postoperative days, and those sacrificed at 150 days underwent another echocardiogram before being slaughtered.
The examinations were performed using a Hewlett-Packard Sonos 5500 apparatus, making every effort to view the pulmonary grafts from different angles, determining the hemodynamic function of the grafts by the mean of the transvalvar gradients and measuring the insufficiency when it existed. The consistency and the thickness of the cuspids and the walls of the conduits were also checked for signs of calcification.
Macroscopic analysis
To remove the graft, the animals were anesthetized similar to the technique used for the initial surgery, sacrificed with an endovenous injection of 19.1% potassium chloride and bled. After left thoracotomy at the third intercostal space and dissection of the pericardial adhesions, the grafts were assessed macroscopically and photographed using a Sony Ciber Shot 32 digital camera. Special attention was paid to the presence of calcium on the wall or the valvar cusp, retraction of the cusps, fenestrations and the presence of thrombi or vegetation.
Radiologic analysis
The grafts were assessed using radiography in a Mammomat C3 from Siemens to detect and analyze the presence and distribution of calcification.
Histological studies
For microscopic analysis, a segment of the arterial wall of the graft containing one of the cusps was fixed in 10% formaldehyde and subsequently embedded in paraffin blocks and sectioned at 4 µm according to conventional techniques. The sections were then stained with hematoxylin-eosin, Gomori's Trichrome and Sirius-red to analyze the collagen fibers under polarized light.
Calcium measurement
To measure the calcium, segments of the wall of the graft and one of the valvar cuspids were separated and digested in 25% nitric acid for 4 hours at 70 ºC and after immersed in a solution of 0.2 N hydrochloric acid. Measurement of the calcium was made in a Perkin Elmer 4100 atomic absorption spectrophotometer using a wavelength of 422.7 nm.
Initial clinical experience
From January 16 to March 19 2003, four patients submitted to the Ross operation had the outflow tract of the right ventricle reconstructed with decellularized valvar homografts. Two patients were male and two female, with ages ranging from 12 to 30 years. The diagnosis was aortic stenosis in two cases and insufficiency in the other two. On two occasions the etiology was rheumatic and the others congenital. The operations were performed using previously described techniques.
Statistical analysis
Results related to the measurement of calcium were compared utilizing the Student t-test, with a p-value of less than 0.05 being considered significant.
RESULTS
It was possible to confirm from the histological control that the cryopreserved valvar homografts maintained their trilaminar structure intact with preserved cellularity, including of the endothelial layer. The cuspids of the decellularized valvar grafts on the other hand, had a complete absence of cellular elements, but with adequate preservation of the extracellular matrix (Figure 1).
All the sheep survived the operation and there were no late mortalities.
At removal of the grafts, it was confirmed that the pericardial adhesions around the decellularized heterografts were less intense than around the cryopreserved homografts. The external macroscopic aspect of the grafts was basically similar in the two groups. There were no areas of significant calcification. Subjectively, however, the decellularized heterografts had a normal consistency in contrast to the cryopreserved homografts which appeared more hardened. The valvar cuspids were thin and translucent, without any sign of thickening, retraction or calcification and with a normal macroscopic aspect in the two groups, with the exception of one cryopreserved homograft at 150 days of evolution that presented with visible calcification in the commissural region.
Also the echocardiographic examinations demonstrated the absence of significant transvalvar gradients in both groups. No signs of thickening or calcification were detected in the examinations.
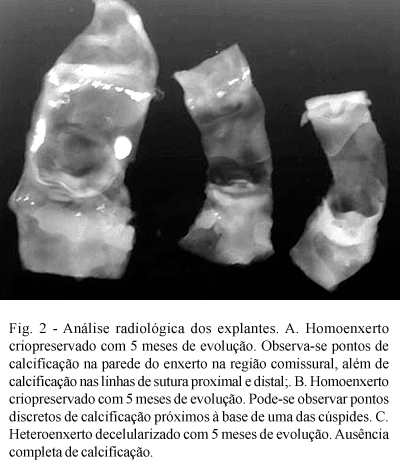
In the radiologic study, however, it was possible to detect areas of calcification on the proximal and distal suture lines in two cryopreserved valvar homografts, but in none of the decellularized heterografts (Figure 2).
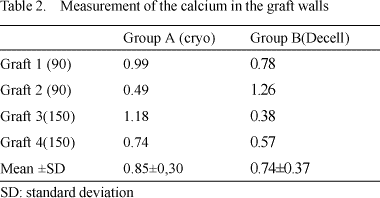
This initial process of calcification can also be demonstrated by the measurement of calcium, as described in Table 1 and 2.
Although the number in the sample is very small for conclusive statistical analysis, the p-value using the student t-test was 0.02, which suggests a lower tendency of calcification of the valvar cuspids of the decellularized heterografts.
With the walls of the grafts, no significant difference between the groups was observed giving a p-value of 0.38.
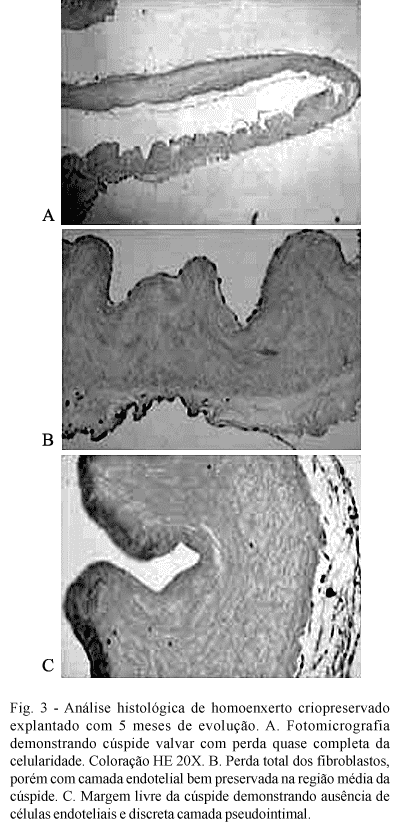
The histological study demonstrated that the cryopreserved homografts suffered a gradual loss of fibroblastic cells, transforming them into an acellular matrix. On some occasions, signs of mucoid degeneration of this matrix was observed. Curiously, the endothelium layer is always preserved, even in grafts with 5 months of evolution. Figure 3 illustrates some histological aspects found in the cryopreserved homografts.
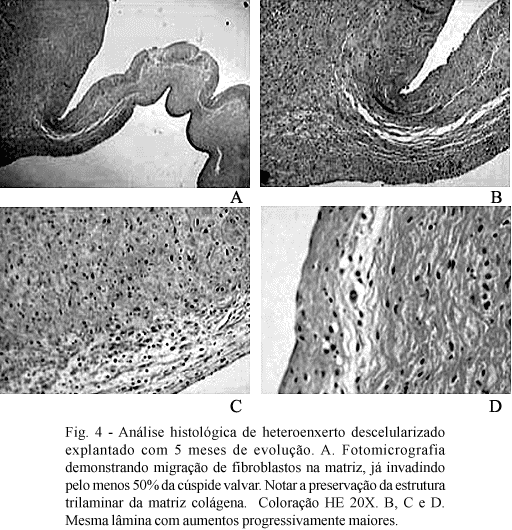
In contrast, decellularized heterografts initially presented with a lymphoplasmocytary reaction, followed by gradual re-population of the acellular matrix. Fibroblastic cells migrate from the base of the cuspid towards the most distal portions, in such a way that after 5 months at least 50% of the surface of the cuspids are repopulated both by fibroblast and by endothelial cells. Eosinophilic prolongation, coming from the tails of the fibroblasts, was very suggestive that these cells are functional in the production of pro-collagen. Different to the cryopreserved homografts, the extracellular matrix maintains its normal trilaminar aspect. On some occasions, islets of fibroblast and endothelial cells in the most distal portions of the cuspids can be seen, which suggest a cellular re-population originating from the blood flow. The histological aspects of the decellularized heterografts are illustrated in Figure 4.
The four patients who underwent surgery had a satisfactory clinical evolution. One of them had to be re-operated on owing to hemorrhage in the immediate postoperative period, with bleeding on the suture line distal to the pulmonary homograft. The control echocardiograms before release from hospital demonstrated normal hemodynamic function of the implanted homografts, with peak gradients always below 10 mmHg and without valvar insufficiency.
COMMENTS
It is imperative that clinical use of any new type of replacement valve is previously submitted to extensive experimental analysis both in vitro and in vivo. Among the many animal models, it is well established that implantation of prostheses in juvenile sheep provides important information not only about the hemodynamic performance, but also about the biological behavior of the different tissues when in the sanguineous current. In the right ventricle outflow tract of juvenile sheep, biological prostheses tend to degenerate and calcify rapidly, enabling inferences about eventual clinical results in humans to be made [19-22].
The data presented here are part of a protocol to develop a new method of preparing valvar homografts, which consists of the reduction of tissue antigenicity by the elimination of the endothelial and fibroblastic cells of the cuspids and arterial wall of the conduit [10].
Valvar homografts have been utilized for the correction of diverse heart diseases for more than 40 years. In this time, several methods of preservation and storing have been used including the use of nutrient solutions with antibiotics, lyophilization, ß-irradiation, ethylene oxide, dry freezing, cryopreservation and even the use of homografts called homovitals. Despite the last two techniques presenting the better results, the late degenerative alterations are still responsible for limited durability of these grafts, especially in children and young adults [1,2].
Although cryopreserved homografts and homovitals present varying degrees of viability of endothelial and fibroblastic cells before implantation, analysis of grafts removed from human beings demonstrated that the valvar cuspids as well as the walls of the conduits are transformed within a few weeks or months, into acellular collagen matrixes. Thus, they do not possess regenerative capacity, remaining subject to the degenerative process [23].
From a hemodynamic viewpoint, the two groups studied here had similar behavior, always demonstrating gradients of less than 12 mmHg and without valvar insufficiency during the observation periods. These data confirm the excellent stability of the non-fixed mature collagen and justify the fact that conventional cryopreserved homografts are still considered to be the gold standard for replacement valves [1,2,11,13].
As opposed to the heterografts fixed in glutaraldehyde, which rapidly calcify in juvenile sheep, the grafts studied here did not demonstrate significant calcification over 150 days of observation, which is in accordance with Jonas et al. [24].
In this study, we can see that, both the valvar cuspids and the walls of the conduits of the cryopreserved homografts presented with a gradual loss of cellularity and the initiation of mucoid degeneration of the extracellular matrix. In some cases, however, some islets of fibroblasts were still present, even in the last grafts to be removed. As opposed to what happens in humans, it is possible to confirm the multifocal presence of a layer of endothelial cells, even in grafts removed after five months of evolution. If this endothelium originates from the donor or if it is re-endothelialization by the recipient, could not be answered by this study. These data confirm the findings of Jonas et al. [24], who observed an integral endothelial layer and complete absence of fibroblasts in fresh and cryopreserved homografts implanted in sheep for periods of up to nine months.
The physiopathologic mechanism of cell death in homografts is still not completely understood. Ramos et al. [25] performed comparative histological analysis of explanted aortic homografts with those of aortic valves of transplanted hearts. They demonstrated that all the homografts were acellular and hyalinized, while the valves of the transplanted hearts maintained their histological structure practically intact, with the presence of endothelium in many foci and presenting only slight reduction of the fibroblasts near to the free borders of the cusps. Studies of the genetic profile of the cells demonstrate that the fibroblasts had origins both from the donor and the recipient. These findings suggest, at least in part, that the cellular destruction in the homografts was caused by an immunological reaction and that the immunosuppressant treatment for transplanted hearts was effective in preserving these cells. However, the possibility that the procedures employed in the preparation of the homografts had altered the extracellular matrix of the cuspids in a way that they had lost the necessary conditions for adherence and cellular proliferation can not be excluded.
Although it is believed that the nutrition of the valvar cuspids is achieved through soaking, Weind et al. [26] demonstrated that the valvar cuspids possess their own sanguineous irrigation. Thus, tissue ischemia in valvar homografts could be implicated in the loss of cellular viability.
Another hypothesis suggests that the harmful effects of the diverse methods of preservation and storing may induce tissue alterations that culminate in cell death. This mechanism can not be proved. Ramos et al. [25] demonstrated that, on the contrary, cryopreserved aortic autografts maintained cellular viability practically integral after implantation with intimal hyperplasia being the only detectable histological alteration.
More recently, tissue engineering has been seen to be promising for the development of several types of grafts. Specifically in the field of heart valve prostheses, a matrix demonstrating in vitro or in vivo re-population by autogenic cells is being sought. This would result in valvar prostheses with regenerative and growth capacity, and thus more physiological and durable grafts [13].
Although many researchers concentrate their efforts to developing a totally synthetic and biodegradable matrix, the utilization of natural matrices such as porcine valves or homografts seem to us to be more appropriate, as they are "natural" matrices. Diverse chemical treatments capable of decellularizing the valvar cuspids and the arterial walls of the conduits have been proposed, including the use of detergents, hypotonic and hypertonic solutions, enzymes such as RNase and DNase, ethanol and deoxycholic acid [11-18].
Elkins et al. [11,13] demonstrated that decellularized porcine heterografts, when implanted in juvenile sheep, had a normal hemodynamic performance for periods of up to 11 months when neither calcification nor tissue retraction was observed. Sequential histological analysis of the grafts demonstrated that soon after implantation an inflammatory reaction caused by tissue invasion basically by macrophages and granulocytes was evidenced, with a small number of lymphocytes. Subsequently, the matrix was invaded by fibroblastic and myofibroblastic interstitial cells, which migrated from the base of the cuspid towards the free margin. After one year of evolution, approximately 80% of the cuspid was repopulated and, by immunohistochemical studies, it was possible to observe the production of type I procollagen suggesting the functionality of these cells. Curiously, re-endothelialization of the grafts was not observed.
Dohmen et al. [12,15] studied decellularized porcine pulmonary heterografts that were re-endothelialized in vitro, which they called pulmonary autoxenografts. When implanted in juvenile sheep, these grafts also demonstrated normal hemodynamic performance, without calcification and without the growth of pannus within the conduit. Additionally, partial re-population of the valvar cuspids by fibroblasts was observed, as was the presence of an integral endothelial layer covering both the surfaces of the cuspids. Because of this, the authors recommend endothelialization in vitro before implantation to assure a more physiological re-population of the grafts.
In this study, we utilized the method of decellularization suggested by Dohmen et al. [10], however, without the in vitro endothelialization. Thus, similar to previously reported studies, we observed a gradual re-population of the valvar cusps, which started at the base of the cusps towards the free margin. These cells seem to originate in the subjacent myocardium or the newly formed vessels in the region of the implantation. Additionally, in two grafts it was possible to see islets of new fibroblasts near to the free margin of the cusps, indicating that blood cells may contribute in cellular re-population. By 150 days of evolution, approximately 50% of the surface was invaded by fibroblasts, similar to what was observed by Elkins et al. [11,13]. Contrary to these authors, however, we observed that our grafts were also partially re-endothelialized, particularly in grafts with longer follow-ups. Perhaps the method of decellularization may explain this difference.
In investigations already started in our laboratory, we questioned about the necessity and advantages of in vitro endothelialization of the grafts before implantation. From a functional point of view, the existence of an integral and complete endothelial layer from the start would be very desirable. Additionally, it is known that endothelial cells have a capacity of migrating to the inside of the matrix and to transform in fibroblastic interstitial cells, which facilitates re-population of the graft. On the other hand, in vitro seeding of endothelial cells is a much more complicated and onerous process with obvious implications in the daily routine [10,12,16].
Although we have not made immunohistochemical studies, histological images suggest that there is production of collagen near to the fibroblasts. Additionally, different to cryopreserved homografts, the collagen matrix seems more intact to us, without signs of mucoid degeneration.
Still in its initial phase, clinical experiments with decellularized grafts are proving to be satisfactory. Elkins et al. [27] recently reported multiple center study with more than 1000 cases of decellularized homografts and heterografts, both in the position of the aorta and pulmonary arteries, in the 4th year of follow-up. Dohmen et al. [28] implanted 70 decellularized homografts and heterografts in the outflow tract of the right ventricle in patients submitted to the Ross operation and in complex congenital heart disease patients with three years of clinical evolution.
Our initial protocol involved the exclusive use of decellularized homografts in the outflow tract of the right ventricle. Our choice of homografts was due to the fact that there are significant interspecies differences in the quality and quantity of the extracellular matrix. Keeping the importance of the extracellular matrix interdependency in mind - cells for which the conditions of growth and cellular functionality are optimal - the use of valvar homografts seems to be, at this moment, more appropriate.
The four patients who underwent surgery had normal clinical evolution with preliminary data of the clinical examination and echocardiograms very satisfactory. For sure, only after a longer clinical follow up will more definite conclusions be reached.
CONCLUSIONS
Decellularized heterografts implanted in the outflow tract of the right ventricle of juvenile sheep gave a satisfactory hemodynamic performance over periods of up to 5 months of follow up.
The explants demonstrated that the cusps were well preserved, without signs of degeneration or calcification. A gradual re-population of the extracellular matrix by fibroblasts and endothelial cells was observed macroscopically.
It is expected that decellularized valvar heterografts and homografts presented a better long-term durability when compared to conventional homografts. Without doubt, the possibility of implanting really viable valves, with a regenerative capacity, represents a great advance in the surgical treatment of valve diseases, having an important impact on the results.
BIBLIOGRAPHIC REFERENCES
1. Bodnar E, Ross D. Valvular homografts. In: Bodnar E, Frate R. Replacement cardiac valves. New York: Pergamon Press 1981;p.287-306.
2. O'Brien MF, Harrocks S, Stafford EG, Gardner MA, Pohlner PG, Tesar PJ et al. The homograft aortic valve: a 29-year follow-up of 1022 valve replacements. J Heart Valve Dis 2001; 10: 334-45.
[ Medline ]
3. Schoen F, Levy R. Calcification of bioprosthetic heart valves. In: Bodnar E, Frate R. Replacement cardiac valves. New York: Pergamon Press 1981;p 125-148.
4. Elkins RC, Lane MM, Capps SB, McCue C, Dawson PE. Humoral immune response to allograft valve tissue pretreated with an antigen reduction process. Semin Thorac Cardiovasc Surg 2001; 13(4 suppl 1):82-6.
5. Dohmen PM, Ozaki S, Yperman J, Flameng W, Konertz W. Lack of calcification of tissue engineered heart valves in juvenile sheep. Semin Thorac Cardiovasc Surg 2001; 13(4 suppl 1):93-8.
6. Schutz A, Fischlein T, Breuer M, Haushofer M, Uhlig A, Detter C et al. Cytoimmunological monitoring after homograft valve replacement. Eur J Cardiothorac Surg 1994; 8:609-12.
[ Medline ]
7. Johnson DL, Rose ML, Yacoub MH. Immunogenicity of human heart valve endothelial cells and fibroblasts. Transplant Proc 1997; 29:984-5.
[ Medline ]
8. Smith JD, Ogino H, Hunt D, Laylor RM, Rose ML, Yacoub MH. Humoral immune response to human aortic valve homografts. Ann Thorac Surg 1995; 60(2 suppl):127-30.
9. Welters MJ, Oei FB, Witvliet MD, Vaessen LM, Cromme-Dijkhuis AH, Borges AJ et al. A broad and strong humoral immune response to donor HLA after implantation of cryopreserved human heart valve allografts. Hum Immunol 2002; 63:1019-25.
[ Medline ]
10. Dohmen PM, Costa FD, Costa IS, Konertz W. Valvas cardíacas obtidas por engenharia de tecidos: a mais nova geração de próteses biológicas. Arq Bras Cardiol 2002; 79:555-9.
[ Medline ] [ Lilacs ] [ SciELO ]
11. Elkins RC, Dawson PE, Goldstein S, Walsh SP, Black KS. Decellularized human valve allografts. Ann Thorac Surg 2001; 71(5 suppl):S428-32.
12. Dohmen PM, Lembcke A, Hotz H, Kivelitz D, Konertz WF. Ross operation with a tissue-engineered heart valve. Ann Thorac Surg 2002; 74:1438-42.
[ Medline ]
13. Elkins RC. Tissue-engineered valves. Ann Thorac Surg 2002; 74:1434.
14. O'Brien MF, Goldstein S, Walsh S, Black KS, Elkins R, Clarke D. The SynerGraft valve: a new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical Implantation. Semin Thorac Cardiovasc Surg 1999; 11(4 suppl 1):194-200.
15. Dohmen PM, Scheckel M, Stein-Konertz M, Erdbruegger W, Affeld K, Konertz W. In vitro hydrodynamics of a decellularized pulmonary porcine valve, compared with glutaraldehyde and polyurethane heart valve. Int J Artif Organs 2002; 25:1089-94.
[ Medline ]
16. Dohmen PM, Ozaki S, Yperman J, Flameng W, Konertz W. Lack of calcification of tissue engineered heart valves in juvenile sheep. Semin Thorac Cardiovasc Surg 2001; 13(4 suppl 1):93-8.
17. Elkins RC, Goldstein S, Hewitt CW, Walsh SP, Dawson PE, Ollerenshaw JD et al. Recellularization of heart valve grafts by a process of adaptive remodeling. Semin Thorac Cardiovasc Surg 2001; 13(4 suppl 1):87-92.
18. Dohmen PM, Ozaki S, Verbeken E, Yperman J, Flameng W, Konertz WF. Tissue engineering of an auto-xenograft pulmonary heart valve. Asian Cardiovasc Thorac Ann 2002; 10:25-30.
19. Trantina-Yates A, Weissenestein C, Human P, Zilla P. Stentless bioprosthetic heart valve research: sheep versus primate model. Ann Thorac Surg 2001; 71(5 suppl):S422-7.
20. Herijgers P, Ozaki S, Verbeken E, Lommel AV, Jashari R, Nishida T et al. The no-react anticalcification treatment: a comparison of Biocor No- React II and Toronto SPV stentless bioprostheses implanted in sheep. Semin Thorac Cardiovasc Surg 1999; 11(4 suppl 1):171-5.
21. Ozaki S, Herijgers P, Verbeken E, Meuris B, Ypermann J, Flameng W. Influence of design and fixation pressure on fibrous sheathing in right-sided porcine aortic bioprostheses. Semin Thorac Cardiovasc Surg 2001; 13(4 suppl 1):1-6.
22. Meuris B, Ozaki S, Herijgers P, Verbeken E, Flameng W. Influence of species, environmental factors, and tissue cellularity on calcification of porcine aortic wall tissue. Semin Thorac Cardiovasc Surg 2001; 13(4 suppl 1):99-105.
23. Koolbergen DR, Hazekamp MG, Heer E, Bruggemans EF, Huysmans HA, Dion RA et al. The pathology of fresh and cryopreserved homograft heart valves: an analysis of forty explanted homograft valves. J Thorac Cardiovasc Surg 2002; 124:689-97.
[ Medline ]
24. Jonas RA, Ziemer G, Britton L, Amiger LC. Cryopreserved and fresh antibiotic sterilized valved aortic homografts conduits in a long-term sheep model. Hemodynamic, angiographic and histologic comparisons. J Thorac Cardiovasc Surg 1988; 96:746-55.
[ Medline ]
25. Ramos T, Neves J, Gulbenkian S, Monteiro C, Santos R, Martins A et al. The allograft valve in aortic valve replacement and in transplanted patients. Cardiac Valve Allografts:1997:109-22.
26. Weind KL, Ellis CG, Boughner DR. The aortic valve blood supply. J Heart Valve Dis 2000; 9:1-8.
[ Medline ]
27. Elkins R. The clinical experience with the SynerGraft. In: 39th Annual Meeting of the Society of Thoracic Surgeons; 2003; San Diego. Proceedings. San Diego: Society of Thoracic Surgeons;2003.




 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license