Background: Dual Chamber Implantable Cardioverter Defibrillator (ICD) in addition to providing hemodynamically improved bradycardia support can also reduce the incidence of inappropriate ventricular shocks owing to episodes of Supraventricular Tachycardia (SVT), with reported incidence of up to 41% of patients. An atrio-ventricular (AV) discrimination algorithm is needed in dual chamber implantable cardioverter-defibrillators (ICD) to avoid these inappropriate shocks.
Methods: An AV discrimination algorithm, SMART DetectionTM, has been developed to differentiate between episodes of ventricular tachycardia (VT) and SVT. It relies on the concept that the chamber with the higher rate is the origin of the tachycardia. The ventricular rate stability criterion is also used to detect concurrent VT during an episode of SVT. Other parameters used in the discrimination algorithm are: atrial and ventricular rhythm multiplicity, P-R regularity, sudden onset. The SMART DetectionTM algorithm has been implemented in the Biotronik Phylax® AV dual chamber ICD. The original SMART DetectionTM has been improved recently with the addition of active detection in the case of 1:1 AV conduction.
Results: 10 patients were implanted with Phylax® AV's at the Bakoulev Institute. Analyses of stored dual chamber intracardiac electrogram (IEGM) indicate that all episodes of VTs and Ventricular Fibrillations (VF) were terminated following appropriate therapies. No episode of Atrial Flutter (Afl) or Atrial Fibrillation (AF) was treated even though the majority of patients have a history of SVTs. In one patient, an episode of Sinus Tachycardia (ST) was inappropriately treated. Increasing the sudden onset criterion, which is used only in the case of stable 1:1 rhythms, was sufficient to prevent recurrence of inappropriate therapy. To avoid the necessity of setting the, not very reliable, sudden onset criterion an active detection procedure was developed. Isolated Premature Ventricular Stimuli were used to test the hypothesis of VT with retrograde atrial conduction in the 1:1 AV rate case. Results of acute tests of this active detection improvement are reported in this paper.
Conclusion: The SMART DetectionTM algorithm, with is active detection improvement in the case of 1:1 AV rate, should prove to be an effective AV discrimination algorithm in dual chamber ICD which will offer freedom from unnecessary shocks in response to SVTs.
Introdução: Cardioversores-desfibriladores implantáveis (CDI) de câmara dupla, além de garantirem um suporte hemodinâmico de bradicardia mais eficaz, também reduzem a incidência de choque ventricular inadequado devido a episódios de taquicardia supraventricular (TSV), cuja incidência na literatura é de até 41% de pacientes. Um algoritmo atrioventricular (AV) discriminatório faz-se necessário em um CDI de câmara dupla, a fim de evitar estes choques inadequados.
Metodologia: Um algoritmo AV discriminatório, o SMART Detection®, foi desenvolvido para diferenciação entre os episódios de taquicardia ventricular (TV) e TVS. Seu mecanismo baseia-se no fato de que a câmara com índice mais alto é a de origem da taquicardia. O critério de razão de estabilidade ventricular é usado também para detectar TV simultânea a um episódio de TSV. Outros parâmetros usados no algoritmo discriminatório são: multiplicidade rítmica atrial e ventricular, regularidade P-R, início repentino. O algoritmo SMART Detection® foi implementado no Biotronik Phylax® CDI AV de duas câmaras. O SMART Detection® original foi aperfeiçoado recentemente com o acréscimo de um detector ativo em caso de condução AV 1:1.
Resultados: O Phylax® AV foi implantado em 10 pacientes no Instituto Bakoulev. A avaliação de eletrogramas intracardíacos (EGMI) de câmara dupla armazenados indicam que todos os episódios de TVs e fibrilações ventriculares (FV) tiveram seu término após terapias adequadas. Nenhum episódio de flutter atrial (FA) ou fibrilação atrial (FA) fora tratado, apesar dos antecedentes históricos da maioria dos pacientes incluírem TSVs. Um único caso de taquicardia sinusal (TS) foi tratado inadequadamente. Ampliando o critério de início repentino, usado somente no caso de ritmos 1:1 estáveis, fora o suficiente para prevenir a repetição de tratamento inadequado. A fim de evitar o uso não muito confiável do critério de início repentino, desenvolveu-se um detector ativo de procedimento. Estímulos ventriculares prematuros isolados foram usados para testar a hipótese de TV com condução atrial retrógrada em casos AV de índice 1:1. Resultados de testes agudos do aperfeiçoamento do detector ativo são relatados neste estudo.
Conclusão: O algoritmo SMART Detection®, juntamente com seu aperfeiçoamento de detecção ativo em casos AV de índices 1:1, deve provar ser um algoritmo AV discriminatório eficaz em CDI de câmara dupla, evitando choques desnecessários devido a TSVs.
INTRODUCTION
The reported incidences of inappropriate shocks in patients with single chamber implantable cardioverter defibrillator have been as high as 41% (1-3) following episodes of Supra Ventricular Tachycardia (SVT). Not only these inappropriate therapies are painful to the patients, but they may even be proarrhythmic (4). While it is possible, from the Holter information collected form these shocks, to adjust the implantable defibrillators to avoid most of them, the patient has already been subjected to unnecessary pain. Even with the best adjustments, a few patients will still suffer from inappropriate shocks (5). Furthermore, there is always the possibility that the adjustment may lead to delayed detection of Ventricular Tachycardia episodes. Thus, it would be highly desirable to have a detection algorithm with a set of easily programmable parameters that can achieve reliable atrioventricular discrimination. With such a detection system, ventricular therapies will be withheld when an episode of SVT is detected. This is especially helpful to patients since most of these supraventricular episodes are of short duration any way, and not life threatening. With the availability of dual chamber defibrillators (6 - 9), such a discrimination algorithm is needed to support the development of atrial antitachyarrhythmia therapies.
The atrioventricular discrimination algorithm, in addition to being able to withhold ventricular therapy, must also be capable of delivering ventricular therapy should an episode of ventricular tachycardia occur in the middle of an on-going supraventricular tachycardia episode.
MATERIAL AND METHODS
The SMART DetectionTM algorithm was developed based on the premise that.
1 the chamber with the higher rate is the origin of the tachycardia:
1 thus if the ventricular rate is the higher rate, then a diagnosis of VT is made
1.2 if the atrial rate is higher, then a diagnosis of non-VT is made
2 with the following special cases:
2.1 if the atrioventricular rate is not exactly 2:1, 3:1, 4:1,... (atrial flutter) and the ventricular rhythm is stable, then we have a concurrent ventricular tachycardia during an episode of supraventricular tachycardia... diagnosis of VT.
3 If the two rates are equal and the ventricular rhythm is stable and:
3.1 the atrial rhythm is unstable, we have a diagnosis of VT
3.2 the atrial rhythm is also stable, we have a number of possibilities that includes ventricular tachycardia with retrograde atrial conduction, atrial tachycardia, sinus tachycardia, junctional tachycardia. Sudden onset is used here to differentiate. Thus if the patient experience a sudden onset in the heart rate, we have a diagnosis of VT.
4 If the two rates are equal and the ventricular rhythm is unstable with irregular PR conduction, we have a diagnosis of VT.
The diagnosis is made on every measured R-R intervals. Each time an R-R interval is classified as VT, a counter is increased. Each time an R-R interval is classified as non-VT, the counter is decremented by a fraction, nominally 1/4. When the counter reaches its programmed threshold, a "VT" diagnosis is declared and ventricular therapy is initiated.
The SMART DetectionTM was implemented in the Biotronik Phylax® AV dual chamber implantable cardioverter defibrillator.
The robustness of SMART DetectionTM comes from a combination of the use of the classification algorithm on each R-R interval and the use of a programmed number of samples meeting the VT classification. Thus, a few mistakes in classification do not jeopardize the final diagnosis.
The classification algorithm used in SMART DetectionTM is not new. Good atrioventricular discrimination has been reported for similar algorithm (9). The only case where the performance has not been good in these earlier studies in the 1:1 stable case, our branch 3.2. The use of the classification on a beat by beat basis, combined with a counter is new.
The "sudden onset" criterion, which has been reported not to be a reliable criterion, is used in SMART DetectionTM only sparingly - only in the case of 1:1 stable rates. It has been recognized from the beginning that the 3.2 branch of the algorithm may not be reliable since atrioventricular nodal e re-entry tachycardia, atrioventricular re-entry tachycardia, triggered atrial tachycardia and ventricular tachycardia with retrograde conduction will all be classified as VT. Thus, in the algorithm we have allowed the physician to override the detection of the 3.2 branch through the use of the sudden onset criterion. For patients with known slow ventricular tachycardia with retrograde conduction, then a small value of sudden onset is programmed to yield to a classification of all these R-R intervals as VT. The sudden onset value programmed still needs to be large enough to avoid therapy being delivered during exercise periods (sinus tachycardia). When the patient is known not to have ventricular tachycardia with retrograde, then this branch can be turned off by programming a large value for the sudden onset criterion. Then, all 1:1 stable rhythms will be declared non-VT. Thus, with our limited use of the sudden onset criterion to the 3.2 branch, we can use this familiar criterion for the fine tuning of the detection process.
Unlike ventricular-only detection algorithms that rely heavily on the rate stability criterion for the differentiation between SVT, mostly atrial fibrillation, and VT, the SMART DetectionTM algorithm uses stability only for the detection of concurrent VT and SVT. With the atrial information available, atrial fibrillation can be easily detected with the larger atrial rate.
Still, the requirement for fine-tuning in the 1:1 A-V rhythm case, is contrary to our desire for a simple to program discrimination algorithm. We have since developed an improvement that applies to the 3.2 branch. Instead of using sudden onset, we decided to go to active detection. Two types of active detection were considered: Premature Atrial Extrastimuli (10,11) and Premature Ventricular Extrastimuli. The premature atrial stimulus tests the hypothesis of ST. The premature ventricular stimulus tests the hypothesis that there is a retrograde path from the ventricle back to the atrium. In both cases, we are looking for a disturbance in the rhythm of the opposite chamber.
Preliminary tests with premature atrial extra stimuli indicated that a long burst of atrial stimuli is needed in order to be able to capture the ventricle during an episode of supraventricular tachycardia. Single isolated stimuli from the auricle (atrial appendage) are typically not able to get through to the ventricle during an episode of supraventricular tachycardia. A long burst in the atrium is dangerous since it may initiate an episode of ventricular tachycardia during an episode of supraventricular tachycardia.
On the other hand, the premature ventricular extra stimuli are aimed directly at the hypothesis that we would like to test, namely: Is the tachycardia of ventricular origin and the events in the atrium are just retrograde events? It is especially applicable when the ventricular pacing electrode is placed in the ventricular apex and the retrograde path is through the atrioventricular node. The electrode is then close to the end of Purkinje fibers and any premature stimuli will be able to propagate to the atrium through the atrioventricular node and on to the atrial sensing electrode if the tachyarrhythmia is of ventricular origin. We assume that electrophysiological studies have already led to the ablation of auxiliary pathways. In the case of a supraventricular tachycardia, the stimuli may be able to propagate to the atrioventricular node but is most likely blocked by the supraventricular tachycardia either in the atrioventricular node or in the atrium. Even in the case of sinusal tachycardia, since only a small amount of prematurity is used, the stimuli will likely be blocked in the atrioventricular node by the anterograde P wave.
RESULTS
The Phylax® AV ICD is a 69cc, 109 g, active housing device which can accept two IS-1 bipolar leads for atrial and ventricular pacing/sensing, a DF-1 ventricular shock coil connection and an optional DF-1 atrial/vena cava shock coil connection. A schematic drawing of the devices is illustrated in Figure 1.
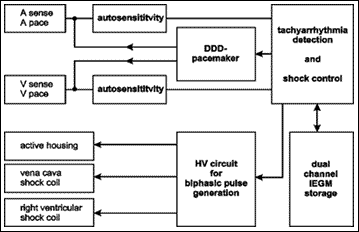
Fig. 1 - Biotronik Phylax AV dual chamber implantable cardioverter defibrillator: schematic drawing.
Ten Phylax® AVs with SMART DetectionTM were implanted at the Bakoulev Institute in the period from April 1996 to July 1997. The characteristics of the patients are summarized in Table 1. The mean follow-up time is 13.3 months ± 4.6 months. With regard to the atrial leads used, 6 passive fixation J leads and 4 active fixation leads were used. For ventricular leads, 3 patients received leads with a ventricular shock coil only, 7 received leads with both proximal (atrium/vena cava) and distal (ventricle) shock coils. An X-ray photograph of one of the last set of patients is shown in Figure 2. All intra-operative tests included:
ventricular fibrillation induction with shock on T, burst with premature extra stimuli, rapid burst
atrial flutter or atrial fibrillation induction by rapid atrial pacing.
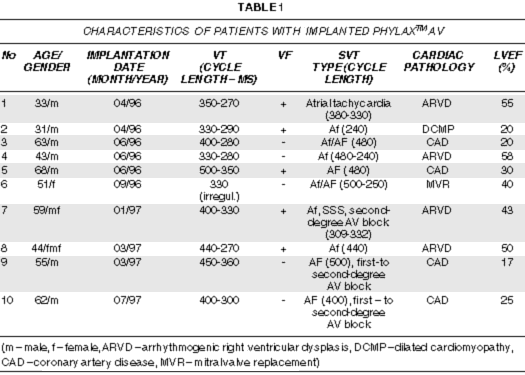
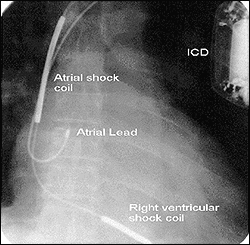
Fig. 2 - Lead system commonly used consisting of an atrial lead and a multi-lumen ventricular lead with two shock coils in the right atrium and right ventricle, and, not shown, pace/sense ventricular tip and ring. The defibrillator housing is another shock electrode.
For the latter tests, we were most frequently successful in inducing Type I atrial flutter with 2:1 atrioventricular conduction. In all cases, the Phylax® AV did not deliver any ventricular therapy. By turning off the SMART DetectionTM feature, essentially reverting the defibrillator to single chamber detection, we were successful in terminating these supraventricular episodes with low energy (1-5 Joules) cardioversion shocks using the standard ventricular shock configuration with the ventricular coil, the defibrillator housing, and if available the atrial shock coil. An example of a Type I (immediate) termination of an episode of Type II atrial flutter (Cycle Length 164-172 ms) is illustrated in Figure 3. This dual channel intracardiac electrogram was stored as part of the diagnostic system of the Phylax AV.
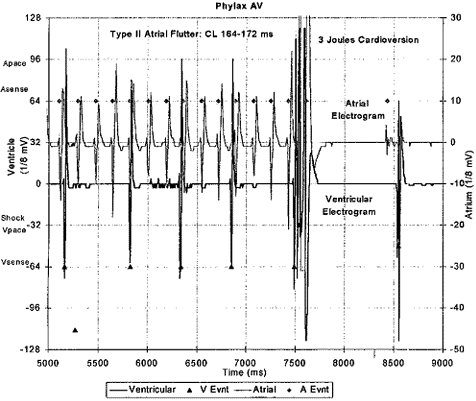
Fig. 3 - Recorded intracardiac stored electrogram of an episode of Type II atrial flutter with 3:1 atrioventricular rate. Episode terminated by a 3 Joule cardioversion shock using the shock coil system shown in Figure 2.
Acute tests of an enhancement of the SMART DetectionTM algorithm using premature ventricular extra stimuli were carried out. PVES were tested in patients with SVT, Wolff-Parkinson-White (WPW) syndrome, VT. A premature ventricular stimulus test is considered to indicate VT if the P-P interval that spans the stimulus becomes different from the previous P-P interval. The results of the PVES tests with three patients are shown in Figure 4. As expected, there is no atrial rhythm variation during an SVT episode. Patients with WPW exhibit disturbances in their atrial rhythm owing to their fast retrograde conduction paths. During VT with retrograde episodes, the VES do manage to perturb the atrial rhythm. However, depending on the timing of the stimulation, not all of these stimulus are able to propagate all the way back to the auricle. The timing of the VES is selected in order to let them coincide with the refractory times of the atrioventricular fascicle (His bundle) based on the detected R-waves, in the case of normal antegrade conduction. This has led to the use of the range of prematurity from 30 ms to 120 ms. Thus if the 1:1 detected rhythm is caused by antegrade waves through the His bundle, the VES (ventricular extra stimuli) will be blocked. Only in the case of retrograde waves, will the VES have the possibility of propagating all the back to the auricle. However, as indicated in Figure 4, not all the stimuli make it all the way to the auricle, probably owing to refractory tissues encountered in the conduction system (cardiac stimulator complex).
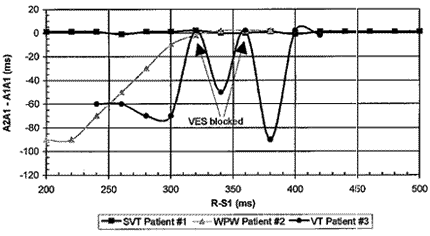
Fig. 4 - Difference of atrial intervals correlated to ventricular interval during ventricular stimulus delivery with decreasing couling intervals for a number of example patients: n - SVT, l - VT, s - WPP.
CONCLUSIONS
The SMART DetectionTM discrimination of the Biotronik Phylax® AV offers a reliable means for the withholding of inappropriate ventricular therapies during episodes of supraventricular tachycardia. This has been verified by the lack of stored intracardiac electrogram recordings with supraventricular episodes. Only episodes of ventricular tachyarrhythmia were recorded. The latest versions of the Phylax® AV offers an "AV success" recording capabilities, when non-treated tachyarrhythmias are recorded. We expect shortly to have improved diagnoses of these supraventricular episodes.
The SMART DetectionTM II discrimination with active detection in the case of 1:1 stable atrioventricular rates should make the programming of these defibrillators even easier. We expect that this will lead to much improved patient comfort.
With a successful atrioventricular discrimination capability, the next step is the combination with atrial therapies leading to a truly dual chamber implantable cardioverter defibrillator.
REFERENCES
1 Hook B G, Callans D J, Hsia H H, Mitra R L - Stored ventricular electrogram analysis in the management of patients with implantable cardioverter-defibrillators. In: Estes III N A, Manolis A S, Wang P J, eds. Implantable cardioverter defibrillators: a comprehensive textbook. New York: Marcel Dekker, 1994: 99-112.
2 Grimm W, Flores B F, Marchlinski F E - Electrocardiographically documented unnecessary, spontaneous shocks in 241 patients with implantable cardioverter defibrillators. Pace 1992; 15: 1667-73.
3 Neuzner J, Pitschner H F, Schlepper M - Programmable VT detection enhancements in implantable cardioverter defibrillator therapy. Pace 1995; 18: 539-47.
4 Cohen T J, Chien W W, Lurie K G et al. - Implantable cardioverter defibrillator proarrhythmia: case report and review of the literature. Pace 1991; 14: 1326-9.
5 Schaumann A, Von Zur Mühlen F, Gonska B D, Kreuzer H - Enhanced detection criteria in implantable cardioverter-defibrillators to avoid inappropriate therapy. Am J Cardiol 1996; 78: 42-50.
6 Schaldach M, Revishvili A S, Merkely B, Lucchese F, Thong T - New concepts and algorithms for dual chamber defibrillators. Biomed Tech 1996; 41: 47-52.
7 Lavergne T, Daubert J C, Chauvin M et al. - Preliminary clinical experience with the first dual chamber pacemaker defibrillator. Pace 1997; 20: 182-8.
8 Rüppel R, Langes K, Kalkowski H, Kühl M, Meinertz T - Initial experience with implantable cardioverter defibrillator providing dual chamber pacing and sensing. Pace 1997; 20: 1078 (Abstract).
9 Revishvili A R, Schaldach M, Thong T - SMART algorithm in dual chamber ICD for supraventricular tachycardia detection. The New Frontiers of Arrhythmias 13th International Congress, Marivella, Italy, January 24-31, 1998. (To be published in Giomale Italiano di Cardiologia).
10 Jenkins J M & Caswell S A - Detection algorithms in implantable cardioverter defibrillators. Proceedings IEEE, 1996; 428-45.
11 Munkenbeck F C, Bump T E, Arzbaecher R C - Differentiation of sinus tachycardia from paroxysmal 1:1 tachycardias using single late diastolic atrial extrastimuli. Pace 1986; 9: 53-64.





 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license