INTRODUCTION
Down syndrome (DS) is a chromosomal abnormality characterized by the extra copy of genetic material on chromosome 21, occurring in whole or in part. It was named after the clinical description by the English physician John Langdon Haydon Down, in 1866 [1]. The worldwide occurrence of DS is estimated at about one to two cases per 1000 births. In Brazil, the estimate of this population was around 300 000 by the 2000 census of the Brazilian Institute of Geography and Statistics (IBGE) [2].
Maternal age after 35 years is a major contributing factor to the occurrence of trisomy 21. Some studies show that advanced paternal age is also a contributing factor [3].
The phenotype of DS is characterized by more than 80 features. The most commom phenotypic manifestations are developmental disabilities, muscular hypotonia, short stature, facial features and congenital malformations, particularly cardiac [4]. The DS is also characterized by disorders and diseases of various organs. These clinical features can vary considerably in number and severity [5]. The phenotype of DS was initially attributed to the loss of chromosomal balance. This hypothesis was weakened by the fact that other autosomal trisomies have no phenotypic variation. Correlations between genotype and phenotype of patients with partial trisomy of chromosome 21 indicate that the restricted region in 21q22.2 is related to the main clinical features of DS. This supports the hypothesis of dose-effect of the gene [6]. In 2007, Yahya-Graison et al. [7] suggest that the heightened expression of these genes would be more related to the phenotype of DS.
The low final height is a major feature of the growth process of DS, and this deficit starts already in the prenatal period [8]. After birth, this speed is much reduced from six to 36 months in both sexes. Puberty usually occurs just prior to the general population and is associated with a deficit of growth [9]. There is also a high prevalence of overweight and obesity, particularly in adolescence and adulthood.
The weight and height growth is a good indicator of health during childhood and adolescence. The different growth and final height of children with DS require the use of curves of weight and height-specific growth [10]. If we put these kids in the graphs of the general population, this may mask the detection of additional diseases such as hypothyroidism, celiac disease and heart disease. Likewise, overweight or obese infant will not be recognized [11,12].
In DS, cardiac malformations occur in 40% to 50% of these children and the most common is atrioventricular septal defect (30% to 60%), followed by ventricular septal defect (30%). Other heart diseases are: ostium secundum atrial septal defect (about 10%), patent ductus arteriosus and tetralogy of Fallot. About twenty years of age, there may be prolapse of the mitral valve with or without tricuspid and aortic regurgitation [13].
Congenital heart disease in the degree of weight-height depends on the type and the same hemodynamic effect [14]. Children with moderate to severe congenital heart disease present more impairment compared to those with mild heart disease or those without heart disease. The improvement of surgical techniques and diagnostic methods in recent decades has allowed to correct heart defects earlier and with better results.
The recovery in weight and stature of these patients postoperatively, within the genetic potential, generates questions and studies. The increased survival and improved quality of life in this population are related to the surgical correction of congenital heart disease. The recoverability of growth of these patients after surgical correction is precisely the main aim of this study.
METHODS
Casuistry
This study was a retrospective cohort study of 181 patients treated in the outpatient clinics of Down Syndrome in the Department of Pediatrics of the Irmandade da Santa Casa de São Paulo in Brazil (ISCSP), by reviewing the records for the period August 20, 1984 to September 19, 2007. The choice of medical records was random or as availability of the medical files at Santa Casa. This study was approved by the Ethics Committee of the ISCSP. All presented with trisomy 21 syndrome diagnosis confirmed by karyotyping and were excluded the mosaics. Of the total records reviewed (n = 181), we excluded patients with serum total tetraiodothyronine (T4) and/or free (FT4) and/or triiodothyronine (T3) changed by age and/or clinical hypothyroidism and leukemia and/or non-cardiogenic factors that could affect the weight and height gain, so the sample reduced to 165 individuals.
The age of patients at the moment of the visit ranged from 0 to 200 months with an average of 49.8 months (±72.1) and median of 6.6 months. Of the total sample (n = 165) were selected those with heart disease and those who were referred for surgery. Subsequently, this group of patients was divided into tertiles, considering the age at cardiac surgery. The first group (G1) included children aged up to 7.56 months at the time of surgical correction. The second group (G2) included those who were between 7.56 and 19.49 months and the third group (G3), those who were older than the previous group. All patients underwent cardiac evaluation to determine whether or not he had heart disease.
In patients with heart disease, this diagnosis was confirmed by clinical examination, electrocardiography, chest radiography, echocardiography with color Doppler, and in some cases by hemodynamic study. In all patients in the sample were performed semiannual doses of thyroid hormones and blood test. Annually, assessment sof all patients were required, otolaryngologic and ophthalmologic and in those who did not undergo cholecystectomy were performed non-abdominal ultrasound examinations.
Anthropometric routine of the Service includes own nursing of this outpatient clinics that performs all actions, observing techniques for the accurate measurement of height and weight. This clinic also provides own anthropometric equipment. The birth data were collected for birth records and subsequent data by reviewing the medical records of patients treated at ISCSP. The weight values +were expressed in grams, height / length in centimeters and age of the children were converted into months. Considering the weight and height as variables of uniform distribution around the average, we calculated the Z score. As the range of variation from the average is the standard deviation, this calculation allowed us to establish how far the height and weight of a particular child were the average of the general population. It was also possible to combine the values +of both genders to form a single group of study. Some computer programs automatically perform this calculation, but this can be obtained by the following formulas:
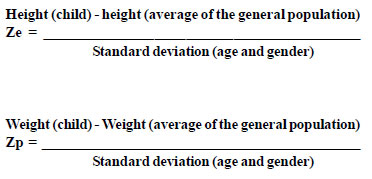
We used in this study the Epiinfo CDC software to calculate the Z score In all patients with a record of weight and length at birth were performed the calculations of Z scores at birth (score Zpn/Zen). Among those who underwent cardiac surgery, these scores were calculated before and after surgery (6 months, 1, 2, 5 and 10 years).
Thus, we determined the involvement of weight and height growth and the time required for recovery of growth after surgery. Statistical analysis performed using the support tool software: BrOffice 3.0, EpiData and EpiData Analysis for Windows/2000 XP.
In all patients who underwent cardiac surgery (n = 60), Z scores were calculated for weight and height before the intervention and the various stages of postoperative described above. Values of continuous variables were expressed as mean and standard deviation. We considered involvement of weight and height growth, score values of weight or height less than -2.5 standard deviations in relation to the general population. The recovery of the Z scores of weight and height was considered as statistically significant difference. To assess the time at which this recovery occurred, we used the difference of means test (Student t test).
To assess whether there was relationship between the recovery of the weight or height with age at operation and in subsequent periods to it, we used the chi-square (
22) Pearson's test. The confidence interval accepted as normal was 95% and was considered as statistical difference when the significance level was
P<0.05).
RESULTS
Of the 165 children selected from the Down syndrome Outpatient clinics in the Department of Pediatrics, at Santa Casa de São Paulo in Brazil, the average age of mothers and fathers (in years) at birth of the child was, respectively, 34. (±6.9) and 36 (±7.03). Age ranged between 16 and 50 years for mothers and between 20 and 55 for fathers with a median of 34 and 36. The Z scores of these children's weight at birth (n = 162) ranged between -5.63 and +1.72, with a mean of -0.95 (±1.2). Z scores for height/length at birth (n = 156) ranged between -6,77 and +0,53, with a mean of -1.34 and a standard deviation of 1.02. The impairment in weight and height of these children with Down syndrome in the general population occurred since birth.
Of the 165 patients with DS, 34.55% (n = 57) had no heart disease (Group 2) and 65.45% (n = 108) had heart disease. Of the total sample (n = 165), 51% (n = 84) were female and ages ranged at the time of the first outpatient care at the outpatient clinics of the Santa Casa of São Paulo, between 0 and 200 months, mean of 30.38 and a median of 6.6 months. The cardiac findings (n = 108) in descending order of frequency were: atrioventricular septal defect (AVSD, 36%/n = 39), ventricular septal defect (VSD, 30.5%/ n = 33), atrial septal defect (ASD; 17.5%/n = 19), associations of heart disease (6.5%/n = 7), patent ductus arteriosus (PDA, 4.6%/n = 5), tetralogy of fallot (T4F, 2.7 %/n = 3) and others (Fig.1).

Fig. 1- Prevalence of types of congenital heart disease in group 1 (n = 108), vertical axis: percentage of heart disease; horizontal axis: types of heart disease; Assoc-associations of one or more heart disease (n=7), ASD - atrial septal defect (n=19), VSD - ventricular septal defect (n=33), AVSD, atrioventricular septal defect (n=39), others, other types of heart disease; PDA - patent ductus arteriosus (n=5) and T4F - Tetralogy of Fallot (n=3)
Of the 108 children with heart disease, 63 (58.3%) were referred to surgery and other (n = 45) had heart disease with mild or no rebound or progressing to spontaneous resolution. Of these 63 children, only 60 underwent heart surgery, because one died before surgical correction, one stopped the care at Service and the responsible of the other patient refused the surgery. The age at the time of surgical correction of heart disease ranged from 1.5 to 168.1 months, with an average of 25 months (±34.6) and median of 10.7 months. Of these children, 58.3% were female. The average Z scores of weight before surgery was -2.62 (±1.35) and mean height Z score was -3.01 (± 1.83). It was observed that 55% of these children (n = 33) had a Zp score below - 2.5 and in relation to height 60% (n = 36) had score values below -2.5. Thus, it indicates the impairment of weight and height in these children with Down syndrome and heart disease with surgical indication, this being more intense commitment to their height.
With six months (6m) after surgery (n = 50) for correction of the heart disease, the mean Z scores of weight and height were respectively -1.95 (±1.17) and - 3.04 (+/-6.09). One year (first) after surgery (n = 40), these mean values of Z scores obtained for the weight and height were -1.32 (±1.14) and of -2.00 (±1.36). With two years (2 nd) postoperatively (n = 34), these mean values of weight Z scores were -0.99 (±1.20) e de -1.58 (±1.16). Five years (5th) after surgery (n 26) (Fig. 2) the means of the weight Z scores were -0.568 (±1.29) and -1.65 (±1.05) (Fig. 2). Ten years (10th) after surgery (n = 13) the mean of height Z score was ± 0.86) and the Z height was -1.55 (= 13), the mean weight Z score was -0.10(±0.86) and for height was -1.55 (± 0.76).
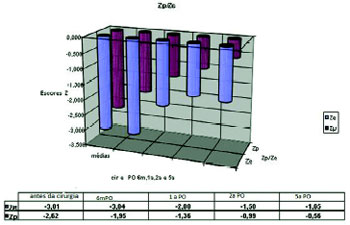
Fig. 2- Distribution of Z scores for weight and length (Zp/Ze) at operation and later periods; the values of Z scores are expressed as means
The Student t test was used to compare the mean scores for height and weight at operation and postoperative periods. The weight recovery after heart surgery, occurred in the majority of this population with Down syndrome six months after the operation, when the Z score of weight (Zp) reached a mean of 1.95 (± 1.17), 67.4% of the sample had a score above -2.5 and the Student T was 4.27 (Table 1).
Regarding the height recovery after surgery, it occurred in most cases (85.7% with Zp over -2.5) at one year postoperatively, when the score (Ze) reached an average of -2.0 (±1.36), 85.7% of these patients achieved a Zp above -2.5 and Student t test was 4.68 (Table 2).
The gradual reduction in the number of cases during the follow-up of the postoperative period (n=60, 6m PO: n=50/ 1
st PO: n=40/2nd PO: n=34/5th PO: n=26 and 10th PO: n=13) occurred for noncompliance of the treatment, the emergence of non-cardiogenic factors (such as hypothyroidism and leukosis) that could jeopardize the growth and death. In the first half, the initial sample was reduced to 50 cases because mortality was higher (6.6%/n=4) and six cases were excluded (factors reported above). In ten years postoperatively, the number of cases was very small (n=13) and statistically insignificant, being excluded from this analysis.
Later, the group was divided by age at the time of surgical correction of heart disease by tertiles. The first group (1) included patients aged up to 7.56 months at operation (33.3% / n=20), the second group (2), patients over age 19 and up to 7.56, 49 (31.7%/n=19) and the third group (3) comprised those who were older than 19.49 months at the time of surgery (35%/n=21). The chi-square test was used to verify whether age is correlated with the height and weight at operation and during the periods subsequent to it. After ten years of operation, the number of patients was not statistically significant, and those cases were excluded from the sample.
P values were calculated by chi-square (X2) Pearson test scores for height and weight at surgery and in subsequent periods. There was no difference in recovery of both height and weight for age at surgery (Tables 3 and 4). The early postoperative mortality was higher in group two who had complete AVSD and secondary pulmonary arterial hypertension (15.7% / n=3). All had previously undergone cardiac catheterization before surgery and presented tests of pulmonary vasoreactivity that allowed this correction.
DISCUSSION
The weight and height growth is one of the most important indicators of child health. Chronic diseases cause impairment of growth, and congenital heart disease are one of the major determinants of such commitment [15]. The development of new surgical techniques and advances in drug therapy has allowed for increased survival and improved quality of life of these patients. The process of recovery of weight and height after surgical therapeutic approach is usual focus of concern for pediatricians, cardiopediatrics and parents. This fact has earned this particular study on a population of children with Down syndrome. In such population, the occurrence of cardiac malformations is common and there are growth characteristics and body composition associated with this syndrome.
Among the well-documented risk factors for the occurrence of meiotic non-disjunction in trisomies, such as chromosome 21, is advanced maternal age at delivery [16]. Mustacchi [17] and others already mentioned authors [3] have also found advanced paternal age as a variable contributing to the occurrence of DS. This study showed a correlation between the occurrence of DS both with advanced maternal age at delivery as advanced paternal age.
Characteristics of the growth of children with Down Syndrome are: a final short stature, reduced growth rate and the tendency to obesity in later childhood and adolescence. The weight and height deficit of children with Down syndrome in the general population initiating in the prenatal period. It was demonstrated in this study that the weight and length were lower from the moment of birth ranging between -0.9 and -1.3. These results were consistent with those of Myrelid et al. [18] where the gaps found at birth ranged between -1.0 and -1.5 for height and between -1.2 and -1.5 for weight. There is some disagreement about the results of Meguid et al. [19] on which these gaps were larger (-1.6 to -3.5 SD) and relative to Cronck [20], with smaller gaps (-0.5 SD).
The occurrence of cardiac malformations in this population is about 40 to 50%, the most common atrioventricular septal defect (AVSD) and ventricular septal defect (VSD) [12,13]. In this study, we found similar results, and heart diseases most frequently encountered were AVSD and VSD. The incidence of girls with heart disease (58.3%) was slightly higher compared to boys as it is described in the literature on population samples with DS [21].
There is consensus that in the general population the majority of children with congenital heart disease have impaired weight and/or height, varying with the severity and the type of heart disease. Heart diseases with increased pulmonary blood flow are those that occur with more severe impairment of the weight of body height. There are few studies on the growth of children with Down syndrome with congenital heart disease, occurring controversial [18,22].
In this study, we observed impaired weight and height of children with heart disease with surgical indication, which, mostly with heart diseases with increased flow. Before operation, 55% (n=33) had a Zp score below -2.5 and in relation to height Z score (Ze), 60% (n=36) had values less than -2.5. The impairment of height was greater than the weight before the operation.
The height and weight recovery after surgical treatment of heart disease occurs in all types of congenital heart disease in the general population [23]. The degree of recovery is controversial. There are few studies in this area, involving mostly a short follow-up of postoperative followup [24]. In the general population, studies show that the postoperative period required for recovery can vary between six months and one year [25] as in this study. The weight recovery was observed in most cases after six months when 67.4% of this sample presented values of Z scores of weight (Zp6m) above -2.5. In relation to height, such recovery occurred in most cases (85.7% with values greater than -2.5) at one year postoperatively. The values of Z scores were compared with the general population because there are no specific formulas that use the average weight / height of the population with DS and no specific growth curves for children with Down syndrome and heart disease, which is a limitation of the study.
In the general population, weight-height recovery after heart surgery is often higher in the surgery before the age of three. In this group of patients the preoperative impairment weight is usually higher, varying the type of heart disease [25]. In this study by dividing the total sample into tertiles according to age at operation, we found the same pattern of recovery. In this sample of patients with DS was observed discordance in the general population according to age at the time of surgical correction of heart disease. We must consider that the population of DS with the most frequent heart disease (AVSD) is a hyperflow, cyanosis and, in most cases with surgical indication in the first year of life.
In agreement with the literature, the children of the group two who were suffering from total AVSD with progression to pulmonary vascular disease had increased intraoperative mortality. It is recommended that children with DS and AVSD is total surgical correction before the first 12 months of life, preferably between three and six months of life and before the onset of pulmonary arterial hypertension.
CONCLUSIONS
Children with DS have impaired growth in relation to the general population from birth.
Congenital heart disease with surgical indication determined a higher weight-height impairment in 55% of the sample of patients with DS for weight and 60% for height.
The recovery of weight and height after surgical correction of this population occurred in most cases in six months for weight, and one year for their height. This impairment is more pronounced for height since birth.
There was relationship between weight gain during the postoperative period and age at operation.
ACKNOWLEDGEMENTS
The National Council of Research and Development (CNPq) and the team at DS outpatient clinics of Santa Casa de São Paulo.
REFERENCES
1. Dunn PM. Dr Langdon Down (1828-1896) and "mongolism". Arch Dis Child. 1991;66(7 Spec No):827-8.
2. IBGE (Instituto Brasileiro de Geografia e Estatística). Censo Demográfico 2000. Disponível em: (27 fev 2009).
3. Zhu JL, Madsen KM, Vestergaard M, Olesen AV, Basso O, Olsen J. Paternal age and congenital malformations. Human Reprod. 2005;20(11):3173-7.
4. Decoq P, Vinckier F. Le syndrome de Down: 1. aspects médicaux. Rev Bélg Méd Dent. 1995;3:43-53.
5. Lyle R, Gehrig C, Neergaard-Henrichsen C, Deutsch S, Antonarakis SE. Gene expression from the aneuploid chromosome in a trisomy mouse model of Down syndrome. Genome Res. 2004;14(7):1268-74.
6. Mao R, WangX, Spitznagel EL Jr, Frelin LP, Ting JC, Ding H, et all. Primary and secondary transcriptional effects in the developing human Down syndrome brain and heart. Genome Biol. 2005;6(13):R107.
7. Aït Yahya-Graison E, Aubert J, Dauphinot L, Rivals I, Prieur M, Golfier G, et al. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact of disease phenotypes. Am J Hum Genet. 2007;81(3):475-91.
8. Clementi M, Calzolari E, Turolla L, Volpato S, Tenconi R. Neonatal growth patterns in a population of consecutively born Down syndrome children. Am J Med Genet Suppl. 1990;7:71-4.
9. Toledo C, Alembik Y, Aguirre Jaime A, Stoll C. Growth curves of children with Down syndrome. Ann Genet. 1999;42(2):81-90.
10. Santos JA, Franceschinini SCC, Prire SE. Curvas de crescimento para crianças com síndrome de Down. Rev Bras Nutr Clin. 2006;21:144-8.
11. Cremers MJ, van der Tweel I, Boersma B, Wit JM, Zonderland M. Growth curves of Dutch children with Down's syndrome. J Intellect Disabil Res. 1996;40(Pt 5):412-20.
12. Mustacchi Z. Curvas padrão pôndero-estatural de portadores de síndrome de Down procedentes da região urbana da cidade de São Paulo [Tese de Doutorado]. São Paulo: Faculdade de Ciências Farmacêuticas da Universidade de São Paulo; 2002.
13. CDC (Centers for Disease Control and Prevention). Birth Defects. [on line] Available from: (2009 Feb 27).
14. Schuurmans FM, Pulles-Heintzberger CF, Gerver WJ, Kester AD, Forget PP. Long-term growth of children with congenital heart disease: a retrospective study. Acta Paediatr. 1998;87(12):1250-5.
15. Villasís-Keever MA, Pineda-Cruz RA, Halley-Castillo E, Alva-Espinosa C. Frecuencia y factores de riesgo asociados a desnutrición de niños com cardiopatía congénita. Salud Publica de Mex. 2001;43:313-23.
16. Peterson MB, Frantzen M, Antonarakis SE, Warren AC, Van Broeckhoven C, Chakravarti A, et al. Comparative study of microsatellite and cytogenetic markers for detecting the origin of nondisjoined chromosome 21 in Down syndrome. Am J Hum Genet. 1992;51(3):516-25.
17. Mustacchi Z, Rozone G. In: Genética baseada em evidências. [CD-ROM] São Paulo: CID Editora;1990.
18. Myrelid A, Gustafsson J, Ollars B, Annerén G. Growth charts for Down`s syndrome from birth to 18 years of age. Arch Dis Child. 2002;87(2):97-103.
19. Meguid NA, El-Kotoury AI, Abdel-Salam GM, El-Ruby MO, Afifi HH. Growth charts of Egyptian children with Down syndrome (0-36 months). East Mediterr Health J. 2004;10(1-2):106-15.
20. Cronk CE. Growth of children with Down's syndrome: birth to age 3 years. Pediatrics. 1978;61(4):564-8.
21. Freeman SB, Bean LH, Allen EG, Tinker SW, Locke AE, Druschel C, et all. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. 2008;10(3):173-80.
22. Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, e al. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81(1):102-10.
23. Dimiti AI, Anabwani GM. Anthropometric measurements in children with congenital heart disease at Kenyatta National Hospital(1985-1986). East Afr Med J. 1991;68(10):757-64.
24. Vaidynathan B, Nair SB, Sundaram KR, Babu UK, Shivaprakasha K, Rao SG, et al. Malnutrition in children with congenital heart disease (CHD) determinants and short term impact of corrective intervention. Indian Pediatr. 2008;45(7):541-6.



 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license