ABSTRACT
Objective: The aim of this study is to evaluate CMC physical-chemical alterations after thermal sterilization and its efficacy in preventing poststernotomy pericardial adhesions.
Methods: After autoclaving thermal sterilization, thermal sterile Carboxymethyl Chitosan (CMCts) was submitted to physical-chemical analysis. Twelve animals were divided into two groups and underwent pericardiotomy and adhesion induction protocol. Afterward, topic CMCts or saline solution was administered. After 8 weeks, a sternotomy was performed for adhesion score macroscopic evaluation, dissection time and the amount of recalcitrant dissection, and microscopic evaluation.
Results: Physical-chemical analysis showed no difference between CMC and CMCts. A macroscopic analysis showed that the intensity of adhesions was significantly lower in the CMCts group (P=0.007). Dissection time and use of recalcitrant dissection also decreased significantly (P=0.007, P=0.008; respectively). Microscopic results indicated a significant reduction in the epicardium collagen area and in the total epicardium area (P=0.05) and (P=0.03).
Conclusion: The sterilization method did not change Carboxymethyl Chitosan physical-chemical properties. Using barrier bipolymer, such as CMCts, can decrease the intensity of pericardium postoperative adhesions, reducing sternotomy complications in cardiovascular reoperations.
RESUMO
Objetivo: Este trabalho tem como objetivo avaliar alterações físico-químicas da carboximetilquitosana após termoesterilização e sua eficácia na prevenção de aderências pericárdicas pós-esternotomia.
Métodos: Após ser submetida a termoesterilização em autoclave, a carboximetilquitosana termoestéril (CMQte) foi submetida a análises físico-químicas. Doze animais foram divididos em dois grupos e submetidos à pericardiotomia e a protocolo de indução de aderências. A seguir, foi aplicada de forma tópica a CMQte ou solução salina. Após 8 semanas, foi realizada esternotomia e avaliação macroscópica do grau de aderências, tempo de dissecção e quantidade do uso de dissecção cruenta e avaliação microscópica.
Resultados: As análises físico-químicas não mostraram diferença entre a CMQ e CMQte. A avaliação macroscópica mostrou que a intensidade das aderências foi significantemente menor no grupo CMQte (P=0,007). O tempo de dissecção e o uso de dissecção cruenta também apresentaram reduções significativas (P=0,007, P=0,008; respectivamente).
Conclusão: O método de esterilização empregado não alterou as propriedades físico-químicas da carboximetilquitosana. O uso de biopolímeros de barreira como a CMQte pode reduzir a intensidade das aderências pós-cirúrgicas no pericárdio, diminuindo as complicações da esternotomia em reoperações cardiovasculares.
INTRODUCTION
The increasing occurrence of cardiovascular reoperations, which represent 10% to 20% of the procedures in this area today [1,2], is cause for concern about pericardial adhesions. These adhesions substantially increase the risk of cardiac lesions of great vessels or extracardiac grafts during sternotomy, thus contributing to greater morbidity and mortality during re-operations [3-5].
Many methods have been tested in an attempt to reduce adhesions [6-9], but only after the appearance of polymer barriers do the results became more consistent and reproducible [1,5,10,11].
Chitosan is a biopolymer with properties similar to the extracellular matrix, abundant in nature and derived from support tissues of crustaceans, insects and fungi. It shows similarity in the basic molecular structure of hyaluronic acid and is distinguished by its biological properties such as anti-bacterial action and atoxicity [12-14], make it an excellent agent for the prevention of postoperative adhesions. For clinical use, it is essential that Carboxymethyl Chitosan (CMC) be submitted to a sterilization process. Some physical and chemical processes - such as the sterilization temperature - can affect the properties of polymers [15, 16].
The aim of this study is to evaluate the effectiveness of thermal sterile Carboxymethyl Chitosan (CMCts) in the prevention of postoperative pericardial adhesions and possible changes in the physical and chemical CMC properties after sterilization via autoclaving.
METHODS
Animals
12 Large-White pigs were used in this study, with weights ranging from 15 to 20 kg. They were divided into two groups of 6 animals each using stratified randomization generated by the SISA software (Simple Interactive Statistical Analysis), available online at
. The protocol of this study was approved by the Ethics Committee of the Heart Institute of the University of São Paulo. All animals received medical care according to the "Guide for Care and Use of Laboratory Animals", published by National Institutes of Health (NIH publication 85-23, revised 1996) and the ethical principles for the care and use of animals on research established by the Brazilian College of Animal Experimentation (COBEA).
Preparation of thermal sterile Carboxymethyl Chitosan
The CMC powder was produced by Dayang Chemicals CO., China, and submitted to sterilization via autoclaving (BAUMER). The process consisted of 5 minutes of pre-vacuuming, 9 minutes of warming up to 134ºC, 15 minutes of moist heat sterilization at a pressure of 2.1 kgf/cm,2 and afterwards, the CMC was submitted to drying for 10 minutes with dry heat at the same temperature. Fifteen ml of CMCts gel at concentration of 3.2% was prepared for the experiment. The same volume of 0.9% saline solution was used as a control.
The Carboxymethyl Chitosan - both sterile and non-sterile - was submitted to physical and chemical analyses: thermogravimetric analysis, hydrogen (1H) nuclear magnetic resonance spectroscopy, infrared spectroscopy, and hydrogenionic potential measuring.
The process followed methods described in studies by Campana-Filho et al. [17, 18].
Animal Experiment
After 12 hours of fasting, the animals received anesthesia infused with an intramuscular injection of 10 mg/ kg of ketamine (Critália, SP, Brazil) and 0.05 mg/kg of atropine (Citopharma, MG, Brazil). The antibiotic-prophylaxis was performed with veterinary solution containing benzylpenicillin potassium, procaine benzylpenicillin, benzathine penicillin and streptomycin (Pentacilin C®, Fort Dodge, SP, Brazil), administered according to the animal's weight. A venous line was punctured in the ear of the animal in which saline solution was infused at 0.9% (HalexIstar, GO, Brazil) at 3 ml/kg/h for volemic replacement and insensitive losses. All animals were monitored with a 2-lead electrocardiogram during the surgical procedure. After the administration of 10mg/kg of thiopental sodium (Critália, SP, Brazil) and 0.05 µg/kg fentanyl chloride (Critália, SP, Brazil) the positioning of oro-tracheal cannula was performed, and connection to the ventilator was scheduled to deliver a tidal volume of 10 ml/kg and an oxygen fraction of 100%. Anesthesia was maintained with isoflurane 0.5-2% (Critália, SP, Brazil).
After antisepsis with Chlorohex® soap and alcoholic antiseptic solution (JohnsonDiversey, SP, Brazil), the operating area was surrounded by sterile surgical drapes. Right anterolateral thoracotomy was performed in the fifth intercostal space of about 5 cm. Pericardiotomy was performed just before the phrenic nerve and the heart was exposed for pericardium repair of 6 points.
Purse-string sutures using 2-0 polyester thread (Mersilene®, Ethicon, SP, Brazil) were performed in the right auricle, ascending aorta and near the inferior vena cava. The epicardium and the inner surface of the parietal pericardium were submitted to mechanical abrasion, using 10 consecutive movements in the following regions of the heart: right atrium; right ventricle (RV) anterior and inferior sides, and the anterior, lateral and inferior sides of the left ventricle (LV). This abrasive agent consisted of a 280-grit water sandpaper (Adalox® T 223, Norton Abrasives, SP, Brazil), mounted on one edge of a wooden spatula (1.5 x 1.0cm area). The sandpapaer-spatula combination was previously assembled and was taken to the autoclave for sterilization.
Twenty cubic centimeters of autologous blood, acquired by puncturing the right atrium, were applied in the pericardial cavity. The blood stayed in this space for over 30 minutes and was then aspirated.
A polyvinyl chloride (PVC) catheter with multiple fenestrations was implanted by counter-opening the pericardial cavity and positioned so that the holes were in contact with the surface of all heart sides.
The pericardium was closed by a single suture line using a continuous running suture with 4-0 polypropylene thread. Before the closure of thoracotomy, the solutions were injected via the catheter according to randomization. After the administration of the substances, the catheter was removed and its hole was sutured so that there would be no significant escape of the infused solution. The right hemithorax was drained at two intercostal spaces below the incision with a tubular drain with a diameter of 18French and connected to a water-sealed drainage system. Atelectasis were removed by manual inflation of the lungs. The intercostal space used was approximated with three 2-0 cotton threads. The sectioned muscle was sutured with thread 0 chromed Catgut®. The skin was closed with an intradermal suture with 2-0 nylon thread. The animal was identified and maintained under anesthesia. After the animal awoke, the orotracheal tube was removed. The chest drain was removed after 20 min of stopping the outflow of air bubbles in the water-sealed drainage system.
The animal was fed 6 hours after the end of anesthesia and received intermittent doses of intramuscular morphine as an analgesic.
Reoperation and euthanasia
The reoperation was performed eight weeks after the initial surgical procedure. After the same anesthetic protocol - except for the antibiotic-prophylaxis - each animal underwent a median thoracotomy. The intensity of the adhesions were evaluated in six pre-defined areas: anterior, lateral and inferior surface of the ventricles, suture in the right atrium, aortic suture and line closure of the pericardium. The adhesions were graded by their intensity using a score system (Table 1) by an evaluator that was not aware of the group to which the animal belonged.
A severity score (named total score of adhesion) was calculated by adding up the scores of each segment analyzed.
The time from the pericardium opening until the end of the lysis of adhesions was counted using a digital chronometer.
Two cameras (FUJI FINEPIX S9600) were positioned to record images and the subsequent quantification of the absolute number of times that a sharp instrument was used in the lysis of adhesions.
After the lysis of adhesions, euthanasia was performed on the animals with the administration of Thiopental (the dose was previously described), followed by a 20-ml bolus injection of 19.1% potassium chloride.
A fragment of tissue was obtained midway between the superior and inferior vena cava and immersed in 10% paraformaldehyde. This sample consists of the right atrial wall, tissue of adhesions, and parietal pericardium.
Anatomopathological studies
After the usual histological processing, blocks were made of paraffin. In CMCts and control groups, cuts of 5ìm in thickness were obtained and stained with Sirius Red [19.20].
Slides were recoded so that the observer did not recognize the group to which the animal belonged in order to prevent bias during the analysis.
To this end, the slides were evaluated using an optical microscope connected to an image analysis system (Quantimet-Leica, Leica Cambridge Ltd., Cambridge, UK). For the evaluation of the fragments, a 5x magnification objective was used.
During the morphometric evaluation, the thickness of the parietal pericardium and adhesion area were measured, and quantitative analysis of collagen in the epicardium, adhesion and parietal pericardium was performed.
Statistical analysis
The categorical variables are presented as median (min.-max.) and the continuous variables are presented as mean ± standard deviation. The data analysis was performed with GraphPad Prism software, version 5.01. The severity score, the time of dissection, the number of times that a sharp instrument was used in the lysis of adhesions and evaluation of histological parameters were evaluated by the Mann-Whitney test. For non-parametric correlations, the Spearman test was used. A non-linear regression was applied to assess the relationship between the adhesion score, time of dissection and quantity of sharp dissection used.
The statistical significance was considered as P<0.05.
RESULTS
There was one death in the group of animals that received the CMCts, and it was due respiratory infection. An animal in the control group presented extensive laceration of the right ventricle during the median sternotomy, and so that the acquisition of data concerning the time of dissection and quantification of recalcitrant dissection related to this animal was impaired.
Analysis of thermal sterile Carboxymethyl Chitosan
Thermogravimetric analysis
The values and the resulting curves are shown in Table 2 and Figure 1. Samples proved to be very similar, differing only on the humidity content. Samples submitted to sterilization presented a higher water content.
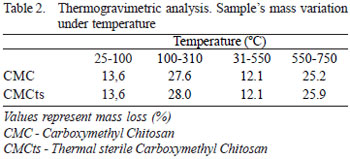
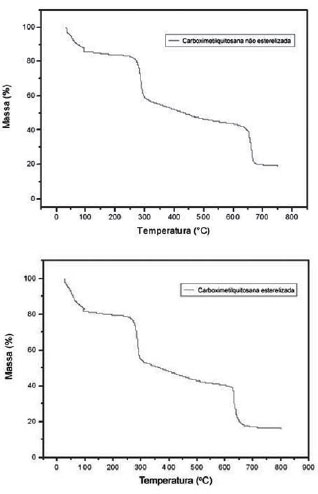
Fig. 1 - Values and curves obtained using thermogravimetric analysis
From this result, the sterile and non-sterilized samples can not be distinguished in terms of decomposition and thermal stability.
Hydrogen (1H) nuclear magnetic resonance spectroscopy
Comparison of the spectra of samples of CMCts and CMC (Figure 2) shows no significant differences. The spectra of the samples also show similarities with the samples of Campana-Filho et al. Also in this figure, the signal to the right (~2.5ppm) has low intensity, which must be due to the three hydrogen atoms of methyl groups of acetamide, indicating that the chitosan used to prepare CMC samples is considerably non-acetylated.
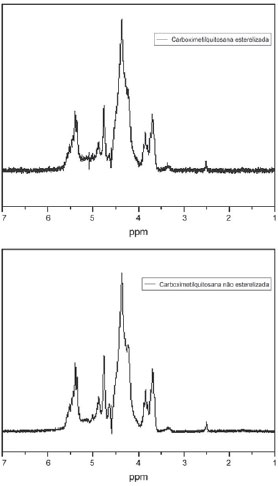
Fig. 2 - Hydrogen (1H) nuclear magnetic resonance spectroscopy
Between 3.5 ppm and 4.0 ppm are the signals attributed to to introduction of one or two groups of carboxymethyl in chitosan amino radicals. The signals due to the introduction of carboxymethyl in chitosan hydroxyl presented above 4.0 ppm and superposed other signals, making it difficult to identify. In spite of this, the sample used can be characterized as O, N-Carboxymethyl Chitosan.
Infrared spectroscopy
Also in this case, it is noted that the spectra are very similar among the samples, indicating the sterilization did not provoke major changes in the samples' structure (Figure 3).
Fig. 3 - Infrared spectroscopy indicating that the sterilization did not significantly change the samples structures
Compared with the samples prepared by Campana-Filho et al. it is known that the samples of Dayang Chemicals presented as sodium, a finding which is reinforced by the intense borders located at about 1410cm-1 and 1600cm-1.
In all spectra, there is a very intense border at about 1100cm-1 that refers to glycosidic linkages among the constituent units of the polymer.
Measuring the hydrogenionic potential (pH)
The pH measurements were performed after magnetic stirring of 4% CMC for 3 days at room temperature. After that period, it was noted that the non-sterile solution (CMC) was yellow and the sterile solution (CMCts) was light brown. In both, the presence of insoluble material in suspension was found. pH-metry was used for the measurements. The results did not differ in the samples, and had a pH at 8.9.
Analysis of the animal experiment
Macroscopic analysis
The analysis results of the intensity of the adhesions (based on the specifications in Table 1) were shown according to each site assessed as median, maximum and minimum values (Table 3). When compared separately, the samples were statistically different for the anterior and inferior side of the left ventricle, as well as the aorta suture line (Mann-Whitney test with P= 0.01).
The result of macroscopic analysis of the intensity of the adhesions with the total severity score is shown in Figure 4. The CMCts significantly reduced the total score of the adhesions when compared to the control group (P=0.007).
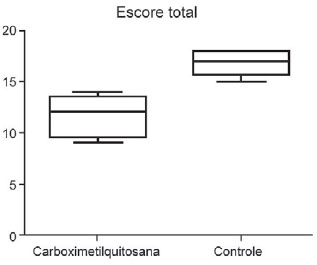
Fig. 4 - Macroscopic analysis of adesions intensity with total gravity score
Quantitative evaluation of dissection time and the use of recalcitrant dissection
The quantitative evaluation of the use of recalcitrant dissection (obtained using the arithmetic mean counting of scissor movements recorded for each camera) showed significant reduction in the group of CMCts. (71.4±23 vs 291±101, P=0.008).
The time of dissection also showed reduction in the CMCts group when compared to the control group (9.8±1.5min vs 33.9±9.2min, P=0.007).
There is a significant correlation between the adhesion score and time spent on the dissection (Spearman r=0.89, P=0.001) and also between the adhesion score and the frequency of recalcitrant dissections (Spearman r=0.87, P=0.001).
The linear regression identifies an exponential relationship between the severity of adhesions and the time of dissection (Figure 5).
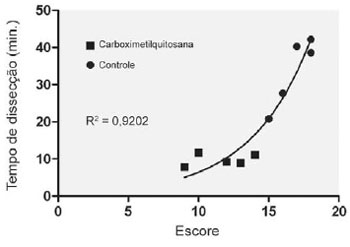
Fig. 5 - Linear regression curve between the severity of adhesions and the time of dissection
Morphometric analysis of histological slides
The morphometric analysis showed that the area of collagen in the epicardium was significantly lower in the CMCts group when compared to the control group (189,900 ± 117,600 µ vs. 440,100 ± 223,000 µ P=0.05), and that the total area of the epicardium presented significant reduction in the CMCts group (652,700 ± 257,500 µ vs. 296,300 ± 183,300 µ P=0.03).
The percentage of collagen was similar in the control group and CMCts in the epicardium, pericardium and adhesion. There was no significant difference between the groups in terms of the area of pericardium and adhesion and the area of collagen pericardium and adhesion, despite the values showing bias reduction in the group CMCts.
DISCUSSION
With the increase in the number of cardiovascular reoperations (50,000 per year in the U.S.) [21], the complications related to sternotomy become increasingly important when evaluating the risk of such a procedure.
Several techniques, such as the closure of the pericardium [7,22] and the use of a heterologous pericardium [6] have already been evaluated to reduce the intensity of adhesions and to consequently reduce morbidity and mortality caused by cardiac lesions, great vessels and extracardiac grafts during sternotomy for cardiovascular reoperations, but without effective or reproducible results.
The use of barrier methods with absorbable biopolymers such as hyaluronic acid, polyethylene glycol, and carboxymethylcellulose have been shown to reduce the intensity of postoperative pericardial adhesions. These methods allow for the repair of mesothelium with visceral and parietal serous separated from each other. Similarly to the results of other authors who used non-sterile CMC [23], the data from this study confirm the effectiveness of this biopolymer - even after sterilization - to significantly reduce the intensity of adhesions.
The biopolymer sterilization process is crucial for its clinical applicability. This study shows that the Carboxymethyl Chitosan thermal sterilization method does not change its main physical-chemical characteristics. When comparing the results found in this study with those from Krause et al. [23] - who used the non-sterile CMC - we noted that both groups found a statistically significant reduction in the adhesion score using this biopolymer. It shows that, despite the physical-chemical characteristics, the CMC biological characteristics were preserved after autoclave sterilization.
There was a death in the group of animals that received the CMCts due respiratory infection, which may have contributed to no statistical difference in morphometric evaluation of the groups when evaluated in the areas of pericardium and adhesions and the area of collagen pericardium and adhesions. The similar elevated percentage of collagen in the groups in all areas suggests that the CMCts do not affect the mesothelial regeneration process, but the formation and structure of adhesions between visceral and parietal pericardium and other structures.
The macroscopic, quantitative use of recalcitrant dissection evaluation and time of dissection indicates a significant reduction in the intensity of adhesions with consequent reduction in the time and risks of sternotomy in cardiovascular reoperations. Although these variables are subjective, the use of an evaluator that was not aware of the animal groupings significantly decreases evaluation bias.
CONCLUSION
Firstly, we can conclude that thermal sterilization does not change the physical-chemical properties of Carboxymethyl Chitosan. We also conclude that the use of barrier biopolymers such CMCts can reduce the intensity of postoperative adhesions in the pericardium, reducing sternotomy complications in cardiovascular reoperations.
ACKNOWLEDGEMENTS
To the Vale do Aço Medical School, students Flávia Luana Barbosa Rodrigues and Pedro Castro Mendes, Veterinary Physician Diva Maria Almeida Oliveira, and laboratory technician Darcy Maria Botelho for cooperation during the study; to Claudia Fajkarz for reviewing the study, and to the São Francisco Xavier Foundation/Usiminas for technical support in the protection of industrial property.
REFERENCES
1. Duncan DA, Yaacobi Y, Goldberg EP, Mines M, O'Brien D, Congdon F, et al. Prevention of postoperative pericardial adhesions with hydrophilic polymer solutions. J Surg Res. 1988;45(1):44-9. [MedLine]
2. Nkere UU, Whawell SA, Sarraf CE, Schofield JB, Thompson JN, Taylor KM. Perioperative histologic and ultrastructural changes in the pericardium and adhesions. Ann Thorac Surg. 1994;58(2):437-44. [MedLine]
3. Bennett SL, Melanson DA, Torchiana DF, Wiseman DM, Sawhney AS. Next-generation hydrogel films as tissue sealants and adhesion barriers. J Card Surg. 2003;18(6):494-9. [MedLine]
4. Garrett HE Jr, Matthews J. Reoperative median sternotomy. Ann Thorac Surg. 1989;48(2):305. [MedLine]
5. Seeger JM, Kaelin LD, Staples EM, Yaacobi Y, Bailey JC, Normann S, et al. Prevention of postoperative pericardial adhesions using tissue-protective solutions. J Surg Res. 1997;68(1):63-6. [MedLine]
6. Gallo JI, Artiñano E, Duran CM. Clinical experience with glutaraldehyde-preserved heterologous pericardium for the closure of the pericardium after open heart surgery. Thorac Cardiovasc Surg. 1982;30(5):306-9. [MedLine]
7. Milgalter E, Uretzky G, Siberman S, Appelbaum Y, Shimon DV, Kopolovic J, et al. Pericardial meshing: an effective method for prevention of pericardial adhesions and epicardial reaction after cardiac operations. J Thorac Cardiovasc Surg. 1985;90(2):281-6. [MedLine]
8. Vander Salm TJ, Okike ON, Marsicano TH, Compton C, Espinoza E. Prevention of postoperative pericardial adhesions. An animal study. Arch Surg. 1986;121(4):462-7. [MedLine]
9. Smith LO Jr. Prevention of surgically induced pericardial adhesions with combined dexamethasone and promethazine therapy. J Fla Med Assoc. 1968;55(5):413-7. [MedLine]
10. Konertz WF, Kostelka M, Mohr FW, Hetzer R, Hübler M, Ritter J, et al. Reducing the incidence and severity of pericardial adhesions with a sprayable polymeric matrix. Ann Thorac Surg. 2003;76(4):1270-4.
11. Robison RJ, Brown JW, Deschner WP, Highes B, King H. Prevention of pericardial adhesions with dextran 70. Ann Thorac Surg. 1984;37(6):488-90. [MedLine]
12. Qian G, Zhou J, Ma J, Wang D, He B. The chemical modification of E. coli L-asparaginase by N,O-carboxymethyl chitosan. Artif Cells Blood Substit Immobil Biotechnol. 1996;24(6):567-77. [MedLine]
13. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457-65. [MedLine]
14. Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol. 1984;28(8):903-12. [MedLine]
15. Jarry C, Leroux JC, Haeck J, Chaput C. Irradiating or autoclaving chitosan/polyol solutions: effect on thermogelling chitosan-beta-glycerophosphate systems. Chem Pharm Bull (Tokyo). 2002;50(10):1335-40. [MedLine]
16. Marreco PR, da Luz Moreira P, Genari SC, Moraes AM. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J Biomed Mater Res B Appl Biomater. 2004;71(2):268-77. [MedLine]
17. Abreu FR, Campana-Filho SP. Preparation and characterization of carboxymethylchitosan. Polímeros. 2005;15(1):79-83.
18. Santos JE, Soares JP, Dockal ER, Campana Filho SP, Cavalheiro ETG. Caracterização de quitosanas comerciais de diferentes origens. Polímeros. 2003;13:242-9.
19. Borges LF, Taboga SR, Gutierrez PS. Simultaneous observation of collagen and elastin in normal and pathological tissues: analysis of Sirius-red-stained sections by fluorescence microscopy. Cell Tissue Res. 2005;320(3):551-2. [MedLine]
20. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447-55. [MedLine]
21. Mayfield WR. Endoscopic repeat sternotomy. Heart Surg Forum. 1998;1(1):26-9. [MedLine]
22. Bailey LL, Ze-Jian L, Schulz E, Roost H, Yahiku P. A cause of right ventricular dysfunction after cardiac operations. J Thorac Cardiovasc Surg. 1984;87(4):539-42. [MedLine]
23. Krause TJ, Zazanis G, Malatesta P, Solina A. Prevention of pericardial adhesions with N-O carboxymethylchitosan in the rabbit model. J Invest Surg. 2001;14(2):93-7. [MedLine]
Article receive on Friday, May 30, 2008








 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license