INTRODUCTION
Research about stem cells (SC) has been continually increasing, and there are promising results for recovering injured tissues, including those of the heart [1,2]. However, the lack of sufficient vasculature (which allows for a fast and appropriate supply of cells infused with oxygen and nutrients) is still a limiting factor for the success of the establishment of tissue engineering and for ischemic tissue treatment [3].
There are currently three types of clinical conditions that could benefit from endothelial progenitor cell (EPC) transplantation: lower limb ischemia, myocardial ischemia, and scarring after heart attacks. Studies have indicated that cell therapy using cells expanded in vitro could promote neovascularization of ischemic tissues, even when applied as a single therapy; that is, without the administration of growth factors [4-6].
A critical limitation of the therapeutic approach with EPCs in the postnatal period is the low number of such cells in circulation. Several studies have been performed in order to optimize the quantity and quality of these cells available for clinical use [4]. Due to the small quantities of EPCs found in peripheral blood, these cells are not readily available for use in clinical trials. Research on the increase of the functional EPC amount may become important.
After myocardial infarction, natural angiogenesis is normally insufficient in meeting the great oxygen and nutrient demands and in preventing apoptosis of hypertrophic cardiomyocytes and ventricular remodeling. Thus, the increase in infarcted myocardium perfusion in order to improve the circulation of oxygen and nutrients by formation of new blood vessels can potentially improve cardiac function [7].
The potential mechanisms for EPCs to induce neovascularization include an increase in the supply of endothelial cells (ECs) through proliferation and EPC differentiation, or an increase in the supply of growth factors to enable the resident mature endothelial cells (ECs). EPCs are CD133+ cells, and most of them co-express CD34 + (only a small percentage is CD34-). Thus, the CD133+ cells represent a subset of CD34+ SC, and are the best population for generating ECs [8]. Sant'anna et al. [9] report on increasing capillaries through gene therapy using a transmural injection of plasmid encoding VEGF 165, which leads to supposed benefits in the reduction and recovery of the ischemic area.
It has already been shown that the human umbilical cord blood (HUCB) contains mesenchymal stem cells [10] and a large number of EPCs [11], suggesting the possibility of using these cells for the revascularization of ischemic diseases. At this point, EPC transplantation derived from HUCBs is still undergoing experiments with animals. The exact population of specific EPCs (isolated, expanded or differentiated) that should be used for transplant has not yet been clearly defined [12].
We have implemented methods to differentiate CD133+ cells in vitro if they have been derived from HUCBs, producing a population similar to the endothelial cells that were assessed in order to prove whether they were functionally active, thus making its future clinical use possible.
METHODS
The experiments were performed at the Experimental Laboratory of Cell Culture - PUCPR, and the Paraná Molecular Biology Institute, with five samples of human umbilical cord blood (HUCB) obtained from the Victor Ferreira do Amaral Maternity Hospital - Curitiba/PR from five participants, who, after receiving all necessary information, signed the written informed consent and agreed to participate in the study. The Research Ethics Committee - Pontifical Catholic University of Paraná (PUCPR) approved this study (number 1366) and it is registered at the Brazilian Research Ethics Commission (CONEP) under title page No. 105806.
Criteria for inclusion of pregnant women donors of HUCBs
Age: 18 to 36 years;
Recorded prenatal care with at least two consultations;
Vaginal delivery or Caesarean section;
Time of amniotic membrane rupture of up to 18 hours;
Parturitions at at least 32 weeks;
Newborn weight of at least 1,500 grams;
Pregnant women without risk behaviors such as drug use, sexual promiscuity, infectious diseases such as hepatitis, sexually transmitted diseases, Chaga's disease or Malaria;
Pregnant women without diseases that may interfere with the placenta's vitality, such as diabetes or hypertension;
Pregnant women who do not use antidepressant drugs, cortcoids, anxiolytics or hormones in general.
Purification and expansion of CD133+ cells
The CD133+ cells were purified using the CD133 MicroBead kit (Miltenyi Biotec ®) linked to anti-CD133 antibody, according to the manufacturer's instructions. The suspension of mononuclear cells (MC) was briefly centrifuged and resuspended in phosphate buffered saline (PBS) (Invitrogen Life Technologies®) supplemented with 5% fetal bovine serum and 2 mM ethylenediaminetetraacetic acid (EDTA). The cells were then filtered (40 µm porosity) and centrifuged for 10 minutes at 450 g. The cell concentration was adjusted to 1 x 10
8 cells (at maximum) in 300 µL of supplemented PBS. We added 100 ìm of blocking solution (Miltenyi Biotec ®) and 100 µm of anti-CD133 antibody (pure) linked to magnetic microBeads (Miltenyi Biotec ®). The material was homogeneized and incubated for 30 minutes at 4 Cº-.
The EPCs resulting from the purification of the MC from HUCB were plated on 12 well-plates covered with fibronectin (BD-Bioscience ®) in supplemented IMDM medium and growth factors (b-FGF) (Invitrogen Life Technologies®), IGF-I (Sigma-Aldrich ®) and VEGF (Sigma-Aldrich ®). After five days of cultivation, the medium was exchanged for the first time and then exchanged again every two days after that. The cultures were kept in a stove, with 5% of CO
2 tension in humid atmosphere. The cells were observed daily using an inverted optical microscope (Olympux IX70 ®) to study their morphology and proliferation. When the cells reached approximately 80% confluence, the cell decoupling was performed using the Acutase enzyme. First the cells were separated from the plates and plated again in cultivation bottles of 25 cm
2 (TPP®), and then separated into two bottles in geometric progression until the 30
th day of cultivation.
Cell characterization by flow cytometry
The characterization, quantification and analysis of the viability of purified and differentiated CD133 + cells were performed using the flow cytometry technique with specific antibodies. The purified cells were analyzed immediately after their isolation, and the differentiated cells were analyzed 30 days after cell dissociation. The technique was performed following Owens et al.13.
The marking was performed with 2 x 10
5 cells that were incubated with various conjugated antibodies and fluorochromes. Isotypes identical to antibodies were used as controls. The acquisition of samples was performed using the FACSCalibur flow cytometer (BD, USA). FlowJo software (FlowJo, USA) was used to perform the analysis.
Tests for evaluating the functionality of the expanded and differentiated cells
Analysis of the VEGF mRNA transcripted expression using RT-PCR
The ribonucleic acid (RNA) of all of the CD133 + purified and differentiated cells was extracted using the RNeasy reagent kit (Qiagen), and following the manufacturer's recommendations. Then synthesis of complementary deoxyribonucleic acid (cDNA) was performed.
The transcripts that encode for the VEGF were amplified by a polymerase chain reaction with reverse transcriptase (RT-PCR) using the cDNA and VEGF primer pair (F1 5CTACCTCCACCATGCCAAGTG3 R1 5TGCGCTGATAGAACATCCATGA3).
The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a constituent and stable gene expressed at high levels in nearly all tissues and cells, and was used as internal control. The amplification products were stained and analyzed by agarose gel electrophoresis at 2%, and the molecular weight marker (1KB Plus) (Invitrogen Life Technologies®) was used. The images were captured by UV
Darkroom (UVP Bioimaging Systems ®), with the Labworks analysis software.
Test of capillary tubules formation in vitro
At the end of 30 days of cultivation, the differenciated CD133+ cells were separated and seeded in 24 well-plates covered with 250 µL of Matrigel
tm (BD-Biosciences®). The cell density was 20,000 cells in 250 µL of supplemented IMDM and growth factors. The cells were incubated in a humidified stove with a tension of 5%, CO
2, and were observed at two, six, twelve and twenty-four hours using an inverted microscope (Olympus IX 70), to verify the formation of capillary tubules in order to prove their in vitro functional capacity. During the examination of cells under the microscope, the images were obtained through the image capture system Spot Insight (Diagnostic).
RESULTS
Cell characterization by flow cytometry
The purified CD133+ cells were immunophenotypically evaluated after isolation with immunomagnetic microbeads coupled to anti-CD133 antibody and the differentiated cells after enzymatic dissociation at the end of the 30-day cultivation (results represented in Figure 1). Thus, it was possible to characterize each of these cell types independently.
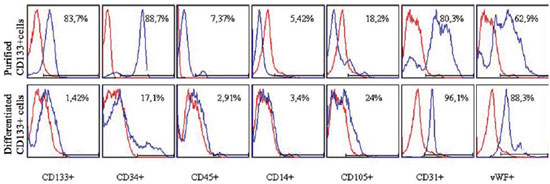
Fig. 1 - Phenotypic characterization by flow cytometry of purified and differentiated CD133 + cells. Cells were marked with anti-CD133, CD34, CD45, CD14, CD105, CD31 and vWF fluorescent human antibodies. Blue histograms identify the percentage of positive cells for each antibody and red histograms identify the isotype controls (negative)
The analysis of VEGF mRNA expression was performed in purified and differentiated CD133+ cells by RT-PCR in five samples. The GAPDH expression was evaluated as internal control, which presented bands for the ten samples. In the purified cells, the presence of transcripts for VEGF mRNA was not verified. Transcriptions for VEGF in all analyzed differentiated cells (Figure 2) were noted. It was shown that only the cells after induction of differentiation began to express the VEGF mRNA transcripts.
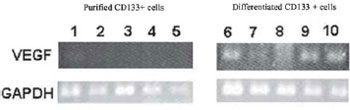
Fig. 2 - Agarose gel of the products of RT-PCR for VEGF and GAPDH. Samples 1 to 5: Purified CD133+ cells; samples 6 to 10: differentiated CD133 + cells
To evaluate the function of differentiated CD133+ cells, the in vitro capacity of these cells to form capillary tubules when plated on MatrigelT was tested. The cells were analyzed and photographed at two, six, twelve and twenty-four hours after plating. The analysis showed that, immediately after plating, the cells had rounded morphology and were randomly scattered on the surface without capillary tubule formation (Figure 3A). And, after 24 hours, they formed tubular structures similar to well-formed capillaries (Figure 3B). In all five experiments (independently performed, and each after 24 hours), the differentiated cells formed capillary tubules
in vitro.
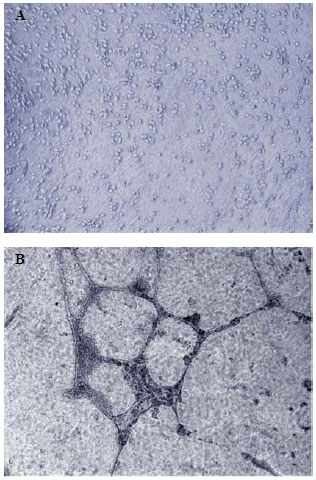
Fig. 3 - Test of capillary tubules formation of CD133+ cells differentiated in vitro. Photomicrography of: (A) cells immediately after plating, presenting rounded morphology and randomly scattered on the surface; (B) cells after 24 hours, showing structures similar to well-formed capillary tubules
Apart from the source, EPCs are difficult to isolate because they represent a very small population when compared with the hematopoietic cells. In peripheral blood, only 0.01% of MCs are EPCs [14]. In bone marrow, less than 0.05% of the cells are EPCs [15]. In previous studies from the authors of this study [16], it was possible to obtain 0.64% of isolated EPCs from HUCB-MC, showing that the HUCB can be a better option as a source of EPCs. This finding is similar to that concluded by Eggermman et al. [15], who showed that the number of EPCs from HUCBs is higher than that found in adult peripheral blood. The exact number of cells needed to successfully induce neovascularization is still uncertain [17]. The possibility of obtaining a greater number of cells may inspire other researchers to complete new testing to determine the more appropriate number of cells needed for this type of therapy.
The cells had their phenotype characterized by flow cytometry. When the cells reached confluence around four weeks, the CD133+ cells were similar to the typical EC phenotype and increased positivity for markers (characteristics for such cells), such as CD31 and vWF [18]. There was a decrease in CD34+ cells. However, some of these cells still remained, according to studies from Gross et al. [14]. Such results also occurred with CD105. There was a significant decrease in CD133 cells, as described by Shmelkov et al. [19]. The number of CD45 and CD14 cells - from hematopoietic and monocytes lines, respectively - was not representative.
The RT-PCR technique clearly showed the presence of VEGF-mRNA transcripts in all five experiments independently performed with differentiated CD133+ cells. It is known that VEGF is decisive in neovascularization function due to its potent mitogenic property for ECs, promoting migration and proliferation of these cells. It also contributes to the remodeling of the extracellular matrix, as well as the formation of capillary tubules and the vascular network [20]. The verification of the presence of VEGF mRNA transcripts is important for confirming that only the EPCs differentiated in vitro possess characteristics that can confirm that such cells are similar to adult EC.
Functional testing through tubular formation on Matrigel
TM showed that the CD133+ cells differentiated in vitro present a formation of structures similar to capillaries, providing additional evidence that the cells proliferated and gave origin to cells similar to endothelial cells. The capacity of cell migration is essential for forming new vessels and capillaries, and it is a characteristic of ECs that are capable of an organization resulting in formation of three-dimensional in-vitro tubular structures [21].
In terms of the functionality of differentiated CD133+ cells, the results seem to be in accordance with a pre-clinical study of Kawamoto et al. [22]. In this study, the EPC differentiated in vitro infused in the ischemic area of rats with acute myocardial infarction were found (through posteuthanasia histological study) in the area of neovascularization. It was shown that these cells not only participate in the induction of paracrine factors - which may stimulate the proliferation of
in situ cells - but also in the physical reconstruction of the lesioned area. In another study, Mukai et al. [23] confirmed the findings of this study, showing that cultivated EPCs could form blood vessels, unlike from EPCs without cultivation, which would promote angiogenesis through the migration and proliferation of mature ECs, suggesting that these two cell populations would have a different role in in vivo neovascularization.
According to Rocha et al. [24], the cells derived from HUCBs are not very immunogenic, which would allow for their use in allogeneic transplant. There is a need for further studies to confirm whether the EPCs differentiated in vitro have HLA antigen expression or not, and what their expression intensity. Even if these cells do not present HLA antigens, other histocompatibility tests must be performed in an attempt to prove whether the EPCs differentiated in vitro trigger an allogeneic immune response. In a study with mesenchymal cells, Cho et al. [25] described that allogeneic mesenchymal cells do not trigger an immune response in normal tissue, but in regions of inflammation; i.e., in the presence of cytokines such as IFN-?-, where rejection can occur. Based on this information, there may be a more solid basis to make viable the use of the differentiated EPCs derived from HUCBs in clinical trials.
The use of EPCs derived from HUCBs can serve as a useful strategy in the study of the nature of these cells before using them in clinical trials. EPCs can be obtained and expanded from HUCBs, and also used for several functions, such as
ex vivo expansion of cells similar to ECs for cellular and gene therapy, or, according to Quirici et al. [26], in
in vitro co-cultures that can provide new perspectives for the treatment of ischemic heart disease. The results obtained by Melero-Martin et al. [27] reaffirm the in vivo therapeutic potential of EPCs to form vascular networks that allow for the vascularization of ischemic tissues and organs.
The performed tests showed that the CD133+ cells differentiated in vitro are similar to ECs in terms of functionality and their potential use in therapeutic applications hereafter .
AKNOWLEDGEMENTS
To Alessandra Melo Aguiar and Patricia Shigunov for performing the molecular biology techniques; to Dr. Vivian Ferreira do Amaral for coordinating the collection of human umbilical cord blood; to Márcia Olandoski for statistical analysis, and to Dr. Alejandro Correa for critical analysis of this study.
This study was supported by the Ministry of Health and the National Council for Scientific and Technological Development (CNPq) - 552233/2005-06.
REFERENCES
1. Schwartz Y, Kornowski R. Progenitor and embryonic stem cell transplantation for myocardial angiogenesis and functional restoration. Eur Heart J. 2003;24(5):404-11. [
MedLine]
2. Scorsin M, Guarita-Souza LC. O transplante celular no tratamento da insuficiência cardíaca. Rev Bras Cir Cardiovasc. 2001;16(3):183-6.
3. Fuchs S, Hermanns MI, Kirkpatrick CJ. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006;326(1):79-92. [
MedLine]
4. Iwami Y, Masuda H, Asahara T. Endothelial progenitor cells: past, state of the art, and future. J Cell Mol Med. 2004;8(4):488-97. [
MedLine]
5. Dallan LAO, Gowdak LH, Lisboa LAF, Schettert I, Krieger JE, Cesar LAM, et al. Terapia celular associada à revascularização transmiocárdica laser como proposta no tratamento da angina refratária. Rev Bras Cir Cardiovasc. 2008;23(1):43-52.
6. Almeida RMS. Carta ao editor: Terapia celular associada à revascularização transmiocárdica a laser: uma nova proposta no tratamento da angina refratária aos métodos terapêuticos. Rev Bras Cir Cardiovasc. 2008;23(2):292-4.
7. Lee MS, Lill M, Makkar RR. Stem cell transplantation in myocardial infarction. Rev Cardiovasc Med. 2004;5(2):82-98. [
MedLine]
8. Chachques JC, Duarte F, Herreros J, Prosper F, Giambroni R, Julia P, et al. Cellular myogenic and angiogenic therapy for patients with cardiac or limb ischemia. Basic Appl Myol. 2003;13(1):29-37.
9. Sant'Anna RT, Kalil RAK, Moreno P, Anflor LC, Correa DLC, Ludwig R, et al. Gene therapy with VEGF 165 for angiogenesis in experimental acute myocardial infarction. Rev Bras Cir Cardiovasc. 2003;18(2):142-7.
10. Kawasaki-Oyama RS, Braile DM, Caldas HC, Leal JCF, Goloni-Bertollo EM, Pavarino-Bertelli EC, et al. Cultivo de células mesenquimais do sangue de cordão umbilical com e sem uso do gradiente de densidade Ficoll-Paque. Rev Bras Cir Cardiovasc. 2008; 23(1):29-34. [
MedLine]
11. Nieda M, Nicol A, Denning-Kendall P, Sweetenham J, Bradley B, Hows J. Endothelial cell precursors are normal components of human umbilical cord blood. Br J Haematol. 1997;98(3):775-7. [
MedLine]
12. Zhang L, Yang R, Han ZC. Transplantation of umbilical cord blood-derived endothelial progenitor cells: a promising method of therapeutic revascularisation. Eur J Haematol. 2006;76(1):1-8. [
MedLine]
13. Owens M, Loken M. Flow citometry principles for clinical laboratory practice. Quality assurance for quantitative immunophenotyping. New York: Wiley-Liss;1995.
14. Gross P, Herbrig K. Role of endothelial progenitor cells in cardiovascular pathology. Rocz Akad Med Bialymst. 2004;49:174-7. [
MedLine]
15. Eggermann J, Kliche S, Jarmy G, Hoffmann K, Mayr-Beyrle U, Debatin KM, et al. Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiov Res 2003;58(2):478-86.
16. Senegaglia AC, Brofman PRS, Aita CAM. Células progenitoras endoteliais de sangue de cordão umbilical humano: purificação, expansão e diferenciação [Tese de doutorado]. Curitiba: Pontifícia Universidade Católica do Paraná;2007. 125p.
17. Zammaretti P, Zisch AH. Adult 'endothelial progenitor cells'. Renewing vasculature. Int J Biochem Cell Biol. 2005;37(3):493-503. [
MedLine]
18. Hoyer LW. The factor VIII complex: structure and function. Blood. 1981;58(1):1-13. [
MedLine]
19. Shmelkov SV, Jun L, St Clair R, McGarrigle D, Derderian CA, Usenko JK, et al. Alternative promoters regulate transcription of the gene that encodes stem cell surface protein AC133. Blood. 2004;103(6):2055-61. [
MedLine]
20. Shen BQ, Lee DY, Cortopassi KM, Damico LA, Zioncheck TF. Vascular endothelial growth factor KDR receptor signaling potentiates tumor necrosis factor-induced tissue factor expression in endothelial cells. J Biol Chem. 2001;276(7):5281-6. [
MedLine]
21. Terranova VP, DiFlorio R, Lyall RM, Hic S, Friesel R, Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985;101(6):2330-4. [
MedLine]
22. Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634-47.
23. Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008;314(3):430-40. [
MedLine]
24. Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342(25):1846-54. [
MedLine]
25. Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, et al. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111(1):430-8. [
MedLine]
26. Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115(1):186-94. [
MedLine]
27. Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761-8. [
MedLine]



 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license