INTRODUCTION
Blood transfusion is related to the occurrence of transfusional reaction, infection transmission, increased postoperative morbidity and mortality, risk for immunosuppression, and hospitalization cost [1,2]. The use of blood components is an independent risk factor for postoperative infection [3]. The use of cardiopulmonary bypass (CBP) can increase postoperative bleeding [4], contributing to the need of blood components. Surgeries without CBP support are related to a significant intra- and postoperative bleeding reduction and, consequently, with the need for hemotransfusion [5].
Several causes expose the patient to the development of major bleeding. Among the predictor factors of the need for hemotransfusion, we can relate: 1. emergency surgery; 2. cardiogenic shock; 3. low BMI (body mass index); 4. severe left ventricle dysfunction (EF<30%); 5. age over 74 years; 6. female; 7. low postoperative hematocrit and hemoglobin; 8. comorbidities (e.g., insulin-dependent diabetes mellitus) and peripheral vascular disease; 9. creatinine > 1,8 mg/dL; albumin < 4g/dL; 10. reoperations; 11. low preoperative PT time; and 12. CBP time.
In this context, CBP has been pointed out as an important potentializer for the occurrence of postoperative bleeding resulting from the increase in coagulation factors consumption, hemodilution, hypothermia, and especially due to inflammatory response [6].
Several strategies have been proposed aiming at reducing postoperative bleeding, consequently minimizing the need of blood and blood components. These strategies comprise the following: 1. The preoperative procedure, beginning with erythropoietin or iron administration weeks before cardiac surgery [7,8] and autologous blood collection before surgical procedure [9]; 2. Strategies adopted during the cardiac surgery, such as reduction in prime volume and the use of equipment such as hemoconcentrators [8], cell saver device [10,11] and Biopump [12], moderate hypothermia (30-32ºC) [8], reinfusion of all blood remaining in the CBP circuit [13], and the use of antifibrinolytics, such as aprotinin, tranexamic acid, and epsilon-aminocaproic acid [14-24], as well as reinfusion of the blood collected by mediastinal drainage in the first six hours postoperatively [13].
Finally, it is admitted that despite the recommended procedures, the main factor to reduce the excessive blood components use can be the adoption of judicious and parametric routines by the surgical teams [2].
The aim of the present study is to evaluate the set of measures adopted by our team in attempting to reduce the need of blood and blood components use, beginning from the decrease of hemorrhagic events, consequently reducing morbidity and mortality, besides their high costs.
METHODS
Between October 2005 and January 2007, 101 consecutive patients were electively operated using CBP support. Of these, 51 were male and 50 female. Patients' age ranged from 13 to 80 years (mean age was 50.76 years). All surgeries were primary and the data collected were reached from a review of patients' medical records.
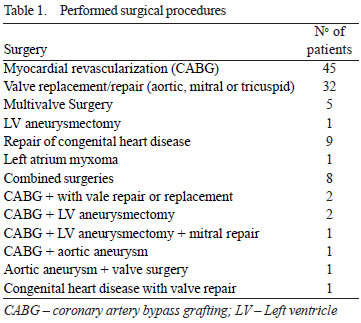
The surgeries performed are shown in Table 1.
Preoperative Hematologic parameters included hemoglobin (Hb), hematocrit (Ht), prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), platelets, and fibrinogen. There was no interruption of acetylsalicylic acid (aspirin) prior to surgery.
Surgical routine
All operated patients were monitored according to service routine (6-channel cardioscopy, mean arterial pressure (MAP), central and peripheral accesses, nasogastric and vesical probe). Antibiotic prophylaxis with 1
st- and 2
nd-generation cephalosporin, corticoid pulse, and general anesthesia with extubation at a specialized intensive therapy unit, all are part of this routine. In all surgeries, the median access route was used. A centrifuge pump (Biopump) was used. Cardioplegia was bloody and isothermal with intermittent administration through aortic puncture or directly into the coronary ostia. Prior to CBP start, heparin 4 mg/kg was administered and the antagonization of this dose was done by protamine sulfate in a ratio of 1:1 after CPB. During CPB, hypothermia was maintained moderately at 30ºC to 32ºC. All patients were taken to ICU with both a transient epimyocardial pacemaker lead and a mediastinum and chest drain (when there was an opening of one or both pleurae).
Employed strategies
1. Antifibrinolytic
The patients undergoing on-pump coronary artery bypass grafting with or without associated procedures received epsilon-aminocaproic acid in a dose of 900 mg in the first hour from the beginning of the anesthetic induction, followed by a dose of 450 mg in the following hours up to 24 hours after surgery completion. Patients undergoing off-pump coronary artery bypass grafting were not given antifibrinolytics. The remaining patients were given tranexamic acid in a bolus dose of 70 mg/kg administered in the anesthetic induction.
2. Normovolemic hemodilution
Normovolemic hemodilution consists in minimizing the prime volume, reducing CPB extension tubes, and using vasoactive amines (adrenalin and noradrenalin) to assist in keeping pressure levels stable during CPB. Perfusate is composed of 500 mL of 0.9 saline solution and 250ml of 20 mannitol. Of this volume, only 20 to 25 mL remains in the membrane oxygenator reservoir. Before CPB is started, a slight arterial hypertension is induced (MAP = 110 mmHg) with the assistance of an adrenalin solution (5 mg of adrenalin diluted in 250 mL of D5W). In this moment, the venous circuit is opened to drain the blood from vena cavae. Blood infusion is started via arterial circuit, when a MAP of 60 mmHg is reached. Throughout CBP, blood pressure is kept within normal limits (MAP: 90 to 100 mmHg); noradrenalin or adrenalin solution is administered, if needed.
During CPB, it is admitted the use of packed red blood cell in the case of hematocrit level is below 20%. The use of fresh plasma in the perfusate is limited to situations, in which preoperative hypoprothrombinemia (TP < 60%) unresponsive to the established measures for normalization of this parameter in the phase of surgery procedures preparation is present. After the aortic unclamping, 20 mL of 10% calcium gluconate and 10 mL of 10% magnesium sulfate are administered.
3. Total perfusate replacement
At the completion of CPB, all the remaining blood into CPB circuit is returned through an arterial cannula. If needed, a sodium nitroprusside solution i9s administered to assist this infusion. The antagonization of the heparin is performed only after the total perfusate volume replacement is achieved (protamine in a ratio of 1:1).
4. Criteria for Administration of Blood Components
After total blood replacement of the CBP circuit, hematocrit and hemoglobin levels are measured. Administration of packed red blood cells is admitted only if these values are below 25 and 8 g/dL, respectively. This same parameter is adopted during the period in which the patient is in the intensive care units and hospitalized. Likewise, the administration of fresh plasma is admitted only if there are cases of prothrombin time impairment, with maintenance of a bleeding volume superior to the acceptable limits (according to reintervention criteria). Cryoprecipitates and platelets are administered if there is an excessive reduction of fibrinogen and platelets, respectively, since there is a persistent bleeding present.
5. Reintervention criteria
We have adopted the most well-known parameters in the worldwide literature. Thus, we have recommended surgical intervention to all patients with persistent blood loss through drain or drains in an average of 3 mL/kg/h (nearly 100 mL/h in a 70 kg patient) in the first two postoperative hours. From the third hour on, if the loss is kept steady in an average of 1.5 mL/kg/h (nearly 100 mL/h in a 70 kg patient), re-exploration is also recommended. Cases in which threshold bleeding occurs, but they course with cardiac buffer evidence (clinical diagnosis by echocardiography) are also surgically re-approached for removal of clot from pericardial cavity and a new hemostasis review.
RESULTS
In 101 surgeries performed, CPB time varied from 15 to 209 minutes (mean of 99.3 minutes). Of these, 27 patients (26.7%) has CPB time superior to 120 minutes.
Baseline hematocrit varied from 30% to 52% (mean of 40.8%), while final hematocrit varied from 23.4% to 45% (mean of 31.5%); of these 66 patients (65.3%) at the end of the surgical procedure had Ht superior to 30% (Figure 1).
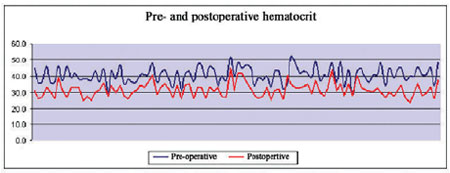
Fig. 1 - Pre- and postoperative hematocrit
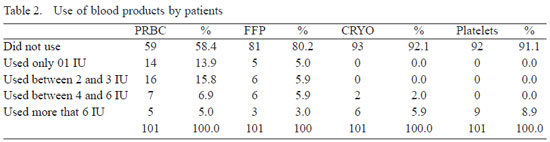
The mean use of blood components in the studied series was of 1.45 IU of PRC/patient; 0,75 IU of FP/patient; 0.89 IU of cryoprecipitate/patient, and 1.43 IU of platelet/patient. In 59 patients (58.4%) there was no need to use blood or blood components and only 12 patients (11.9%) surpassed the limit of four packed red cell units. Eighty-one patients (80.2%) did not use fresh plasma and only 8 patients (7.9%) needed cryoprecipitates, while 9 patients (8.9%) needed platelet concentration (Figure 2 and Table 2).
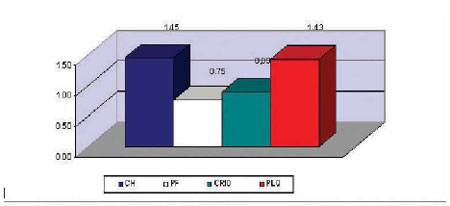
Fig. 2 - Utilization average of blood products
Of the 27 patients (26.7%) whose CPB time surpassed 120 minutes, 17 (63%) needed hemotransfusion. Of the 101 patients, only three (2.97%) developed coagulopathy; of these two patients (1.98%) underwent surgical reexploration due to bleeding. All the aforementioned patients had CPB time superior to 120 minutes. Of the 74 patients (73.3%) whose CPB time was below 120 minutes, 50 patients (67.6%) did not need hemotransfusion and only three patients (4%) were transfused with four or five IU of packed red cells; none of the patients needed more amount of packed red cells.
Of the 101 patients, 26 (25.7%) were elderly (age e" 65 years); of these, eight patients (30.8%) did not need blood products and 10 (38.4%) used up to e IU of packed red cells. On the other side, of the three patients who developed coagulopathy, two were in this subgroup of patients.
Other complications were as follows: acute myocardial infarction (AMI)/4 patients (3.9%); acute renal failure (ARF)/ 4 patients (3.9%); sepsis/8 patients (7.9%); in two of these patients, sepsis was secondary to mediastinitis, resulting in one death and in the other remaining six patients, sepsis was the result of respiratory infection or infection from the urinary tract; SIRS was present in two patients (1,9%); in one of them SIRS manifested itself through coagulopathy (both patients died); stroke/1 patient (0.99%); surgical wound infection/1 patient (0.99%); and pulmonary hypertension seizure/1 patient (0.99%); the patient died. Two patients (1.9%) died of cardiogenic etiology (AMI).
DISCUSSION
We can divide the strategy to reduce blood product in cardiac surgery into some subgroups, which are discussed below.
1. Preoperative strategies
The judicious preparation of the patient preoperatively is an important instrument in attempting to reduce postoperative bleeding and the need of hemotransfusion. Factors related to a higher need of blood products use include: elderly patients, female, high functional class (New York Heart Association), low Ht, reoperation, and high CPB time [3]. Precautions such as a careful investigation of family bleeding history and adequate laboratory assessment (PT, aPTT, platelets, TT, TS, and fibrinogen) contribute to prevent the occurrence of bleeding during CPB [19].
It has been proven that autologous blood collection before surgical procedure reduces the need of blood transfusion [9]. It is a simple procedure that can be performed preferably at least 10 days before surgery, or even after chest opening with a direct drainage through right atrium puncture.
The administration of erythropoietin two to three weeks prior to cardiac surgery is associated with a significant reduction in the need of hemotransfusion [7]. Erythropoietin has been used to increase packed red cell mass and its main side effect is increased systolic hypertension. The routine use of this drug is limited due to its high cost [2]. Patients with religious motivation who reject blood products or present markedly low levels of hematimetric parameters can benefit from the use of this medication. In spite of this, we have restricted its use to specific cases. Serum iron dose and oral iron administration constitute preoperative routine in some hospital services [8].
In the present study, we were not concerned with the withdrawal of preoperative acetylsalicylic acid (aspirin) use, especially when CABG surgery was performed. Some studies have shown that the use of preoperative aspirin therapy does not increase the need of hemotransfusion in elective surgeries, or even in reoperations [20].
2. Intraoperative strategies
2.1 Antifibrinolytic use: Antifibrinolytic use reduces systemic inflammatory response in patients undergoing CPB [16], contributing to the reduction of postoperative bleeding [17,18]. Antifibrinolytics alone [15], or in association with hemoconcentration and re-infusion of all the remaining blood of CPB circuit, as well as the blood drained in the immediate postoperative period reduce the need of using blood products after cardiac surgery [15]. Low-dose aprotinin, epsilon-aminocaproic acid and tranexamic acid when administered to patients receiving aspirin who will undergo CABG reduce the need of blood products and postoperative bleeding [14]. In spite of this, the use of aprotinin is related to side effects [21], especially in patients undergoing CABG. For this reason, we chose to restrict the use of this drug just to reoperation cases. In this context, we have reserved epsilon-aminocaproic acid for CABG cases and tranexamic acid for other surgeries. We chose not to use any antifibrinolytics.
2.2 Normovolemic hemodilution: The reduction of prime volume varies in different studies. Some of them have shown that the volume of 1000 mL of crystalloid solution is efficient in minimizing the hemnodilution [8]. In the protocol followed in our Service, we chose a further reduced prime volume of 750 mL of crystalloid solution, with satisfactory outcomes. Likewise, the re-infusion of all blood of CPB circuit, as well as the blood collected through the drainage of the mediastinum in the first six hours postoperatively have shown significant reduction of blood and blood products use, as well as morbidity and mortality [8,12]. This strategy requires sometimes the association of vasodilator drugs, such as the sodium nitroprusside, to accommodate all the residual volume.
Up to the present moment, we did not use the re-infusion systems of blood collected through mediastinal drain, because most all the time this postoperative bleeding has been sufficiently reduced. Recently, the concept of a minibypass system (reduction of the prime volume, tubing circuit, and vacuum drainage) has shown, especially in CABG surgeries, a reduction of the negative effects of conventional CPB,including the use of blood products [25].
2.3 Devices and equipments: The use of centrifuge pumps (Biopump) allows the reduction of excessive heparin use [11], reducing intra- and postoperative bleeding. Also, the use of hemoconcentrators is related to a reduction of postoperative bleeding [8]. The effectiveness of using cell saver device is controversial. Some studies have shown the benefit and the reduction of hemotransfusion need [10], while others did not find significant benefit [22]. The high cost and the dubious outcomes suggest that the use of cell saver device may not be cost-effective [23]. Some studies have reserved the use of cell saver device for aorta surgeries or reoperations [8]. In our experience, we were able to achieve good outcomes just using centrifuge pumps, thus limiting the high cost of these devices.
2.4 Hypothermia: Maintain temperature during a CBP between 30ºC and 32ºC (moderate hypothermia) is related with reduction of intra- and postoperative bleeding [8]. In the rationale presented, we adopted this routine with satisfactory outcomes.
SOUZA, HJB ET AL - Strategies to reduce the use of blood components in cardiovascular surgery Rev Bras Cir Cardiovasc 2008; 23(1): 53-59 58 Some factors favoring the occurrence of bleeding during CPB are hyperheparinemia, heparin rebound effect, and the excessive protamine use [19]. Thus, the verification of the activated clotting time (ACT) during and immediately after CPB can be an important evaluation tool of these parameters.
Even though it has been described that an hematocrit of 15% can be well tolerated after coronary artery bypass surgery without any sequelae [24], we believe that these indexes increase postoperative morbidity and mortality. This being the case, we can assure that the strategies applied succeed in restrain the use of blood and blood products. The set of measures adopted and other related in the worldwide literature are, up to date, sufficient to drive and to allow the selection of those that can contribute to intraand postoperative blood transfusions reduction. In this sense, we reinforced the idea that surgical teams should have in mind and re-educate themselves aiming at limiting the use of blood products to cases extremely necessary, adopting strictly parameters and routines, but easily reproducible without necessarily increase the cost of the procedures.
CONCLUSION
In the series presented, the measures adopted achieve to substantially reduce the need of using blood and blood products in patients undergoing cardiac surgery. The result presented in the subgroup of patients with CPB time over 120 minutes show a trend of greater need of hemotransfusion if compared to those with lower CBP time. Regarding patients' age, it was not verified any important association between hemotransfusion need and surgery in an elderly patient. However, the association of surgery in elderly patients and CBP time superior to 120 minutes resulted in a larger use of postoperative blood and blood products.
REFERENCES
1. Alghamdi AA, Davis A, Brister S, Corey P, Logan A. Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion. 2006;46(7):1120-9. [
MedLine]
2. Ferraris VA, Ferraris SP. Limiting excessive postoperative blood transfusion after cardiac procedures: a review. Tex Heart Inst J. 1995;22(3):216-30. [
MedLine]
3. Banbury MK, Brizzio ME, Rajeswaran J, Lytle BW, Blackstone EH. Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg. 2006;202(1):131-8. [
MedLine]
4. Hall TS, Brevetti GR, Skoultchi AJ, Sines JC, Gregory P, Spotnitz AJ. Re-exploration for hemorrhage following open heart surgery differentiation on the causes of bleeding and the impact on patient outcomes. Ann Thorac Cardiovasc Surg. 2001;7(6):352-7. [
MedLine]
5. Niranjan G, Asimakopoulos G, Karagounis A, Cockerill G, Thompson M, Chandrasekaran V. Effects of cell saver autologous blood transfusion on blood loss and homologous blood tranfusion requirements in patients undergoing cardiac surgery on-versus off-cardiopulmonary bypass: a randomised trial. Eur J Cardiothorac Surg. 2006;30(2):271-7. [
MedLine]
6. Inada E. Blood coagulation and autologus blood transfusion in cardiac surgery. J Clin Anesth. 1990;2(6):393-406. [
MedLine]
7. Alghamdi AA, Albanna MJ, Guru V, Brister SJ. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg. 2006;21(3):320-6. [
MedLine]
8. Van der Linden P, De Hert S, Daper A, Trenchant A, Jacobs D, De Boelpaepe C, et al. A standardized multidisciplinary approach reduces the use of allogeneic blood products in patients undergoing cardiac surgery. Can J Anesth. 2001;48(9):894-901.
9. Dietrich W, Busley R, Kriner M. Preoperative autologous blood donation in cardiac surgery. Reduction of allogeneic blood requirements. Anaesthesist. 2006;55(7):753-9. [
MedLine]
10. Parrot D, Lancon JP, Merle JP, Rerolle A, Bernard A, Obadia JF, et al. Blood salvage in cardiac surgery. J Cardiothorac Vasc Anesth. 1991;5(5):454-6. [
MedLine]
11. Sellevold OF, Berg TM, Rein KA, Levang OW, Iversen OJ, Bergh K. Heparin-coated circuit during cardiopulmonary bypass. A clinical study using closed circuit, centrifugal pump and reduced heparinization. Acta Anaesthesiol Scand. 1994;38(4):372-9. [
MedLine]
12. Paiva P, Ferreira E, Antunes M. Bloodless open heart surgery: simple and safe. Rev Port Cardiol. 2005;24(5):647-54. [
MedLine]
13. Penta de Peppo A, Pierri MD, Scafuri A, De Paulis R, Colantuono G, Caprara E, et al. Intraoperative antifibrinolysis and blood-saving techniques in cardiac surgery. Prospective trial of 3 antifibrinolytic drugs. Tex Heart Inst J. 1995;22(3):231-6. [
MedLine]
14. Landymore RW, Murphy JT, Lummis H, Carter C. The use of low-dose aprotinin, epsilon-aminocaproic acid or tranexamic acid for prevention of mediastinal bleeding in patients receiving aspirin before coronary artery bypass operations. Eur J Cardiothorac Surg. 1997;11(4):798-800. [
MedLine]
15. Slaughter TF, Faghih F, Greenberg CS, Leslie JB, Sladen RN. The effects of epsilon-aminocaproic acid on fibrinolysis and thrombin generation during cardiac surgery. Anesth Analg. 1997;85(6):1221-6. [
MedLine]
16. Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilon-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003;126(5):1498-503. [
MedLine]
17. Kikura M, Levy JH, Tanaka KA, Ramsay JG. A double-blind, placebo-controlled trial of epsilon-aminocaproic acid for reducing blood loss in coronary artery bypass grafting surgery. J Am Coll Surg. 2006;202(2):216-22.
18. Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briët E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354(9194):1940-7. [
MedLine]
19. Bick RL. Alterations of hemostasis associated with cardiopulmonary bypass: pathophysiology, prevention, diagnosis, and management. Semin Thromb Hemost. 1976;3(2):59-82. [
MedLine]
20. Tuman KJ, McCarthy RJ, O'Connor CJ, McCarthy WE, Ivankovich AD. Aspirin does not increase allogeneic blood transfusion in reoperative coronary artery surgery. Anesth Analg. 1996;83(6):1178-84. [
MedLine]
21. Alderman EL, Levy JH, Rich JB, Nili M, Vidne B, Schaff H, et al. Analyses of coronary graft patency after aprotinin use: results from the International Multicenter Aprotinin Graft Patency Experience (IMAGE) trial. J Thorac Cardiovasc Surg. 1998;116(5):716-30. [
MedLine]
22. Boldt J, Zickmann B, Czeke A, Herold C, Dapper F, Hempelmann G. Blood conservation techniques and platelet function in cardiac surgery. Anesthesiology. 1991;75(3):426-32. [
MedLine]
23. Hall RI, Schweiger IM, Finlayson DC. The benefit of the hemonetics cell saver apparatus during cardiac surgery. Can J Anaesth. 1990;37(6):618-23. [
MedLine]
24. Mathru M, Kleinman B, Blakeman B, Sullivan H, Kumar P, Dries DJ. Myocardial metabolism and adaptation during extreme hemodilution in humans after coronary revascularization. Crit Care Med. 1992;20(10):1420-5. [
MedLine]
25. Perthel M, El-Ayoubi L, Bendisch A, Laas J, Gerigk M. Clinical advantages of using mini-bypass system in terms of blood product use, postoperative bleeding and air entrainment: an in vivo clinical perspective. Eur J Cardiothorac Surg. 2007;31(6):1070-5.



 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license