INTRODUCTION
Bronchopulmonary dysplasia is a multifactorial disease, whose etiology is not completely established yet. It results from the synergism of aggression factors to the immature pulmonary tissue, such as mechanical ventilation, oxygen therapy, infection, PDA (patent ductus arteriosus), prematurity, nutrition, and genetics. Bronchopulmonary dysplasia develops from an acute lesion in an immature lung, resulting in an interruption of the normal process of the lung development with impairment of vascular and alveolar growth followed by the abnormal repair process, with the development of chronic pulmonary disease [1].
The basic evaluation method is still the thorax radiography. However, it presents low sensitivity for some lesions when compared to the high-resolution computed tomography (HRCT), which can show subtle abnormalities, such as septal lines, honeycombing, parenchymal bands, and ground-glass opacity, among others [2,3]. Ground-glass opacity is the increased lung attenuation in which it is still possible to identify the contours of the vessels and bronchial structures within the pulmonary area affected by a pathological process that causes a reduction in the aeration of the air spaces [4].
In up to 80% of the times, extremely low birth weight (ELBW) premature infants, i.e., a birth weight less than 1500 g, can present a patent ductus arteriosus (PDA), with ELBW being inversely proportional to gestational age and birth weight at birth [5]. Such children often require treatment for closure of the patent ductus arteriosus, once this leads to an increased pulmonary blood flow and to an interstitial edema with reduced pulmonary compliance besides an increased airway resistance may lead to both higher mechanical ventilation time and risk of bronchopulmonary dysplasia (BPD) [6].
Procedure for closure of the patent ductus arteriosus can either be clinically performed with a prostaglandin inhibitor which proved to be ineffective in up to 40% of patients, or surgically performed by direct PDA ligation. Such approaches are individualized and, currently, there is no consensus regarding the superiority over one another. This fact may have a direct influence on hospitalization time and in the child prognosis as to pulmonary lesions [7].
In this way, we search to evaluate lately through high-resolution computed tomography the lung parenchyma of the extremely low birth weight (ELBW) premature infants with PDA undergoing surgical or pharmacologic treatment aiming at PDA closure and who presented with bronchopulmonary dysplasia.
METHODS
Between December 2006 and January 2007, 14 children, mean age of 36.5±4.3 month-old, underwent high-resolution computed tomography to study the lung parenchyma. Nine (64.3%) patients were males and five females (35.7%). Mean birth weight was 939.6±288.4 g.
The study included premature infants with gestational age less than 29 weeks and with a very low birth weight (less than 1500 g), who developed bronchopulmonary dysplasia and required PDA closure treatment for over a year. Patients were divided into two groups: Clinical group or group A (n=6) included patients treated with prostaglandin inhibitors only; Surgical group or group B (n=8) included patients with prostaglandin inhibitors failure and required referral to surgical ligation of the ductus or were treated with surgical ligation only.
In group A, median birth weight was 1005 g, ranging from 815 to 1400 g; in group B, median birth weight was 840 g, ranging from 480 to 1380 g.
In the present retrospective study, clinical data were obtained through medical records analysis, gender, age, birth weight, durations of mechanical ventilation (MV), use of oxygen, and admission only.
Table 1 shows intubation time, duration of oxygen dependence, and days of hospitalization. It can be observed that the mean and median values are close to each other, thus disclosing the low influence of clinical cases with atypical and disagreeing values.

Hospital dispensary exams were performed at the clínica radiológica Ultra-X Diagnóstico por Imagem de São José do Rio Preto, using a GE LightSpeed (TM) multi-slice CT system (General Electric Medical Systems, Milwaukee, WI), allowing physicians to view 16 images per second with slice intervals of 0.65 cm. All the patients were sedated with propofol (2.0 mg/kg) administered in a peripheral vein and monitorized by pulse oxymetry and noninvasive mean arterial pressure, and followed-up during the CT scan by a multidisciplinary staff composed of an anesthesiologist, a pediatrician, and a nurse. There was not observed a reduced respiratory depression and saturation, consequently orotracheal intubation was not required. Sedation was spontaneously reversed after withholding drug therapy. Patients were discharged from hospital after 6 hours.
Pulmonary lesions seen on HRCT were described according to the Brazilian Consensus on Terminology Used to Describe Computed Tomography of the Chest, observing areas of low attenuation and reduction in vessel caliber, atelectasis, parenchymal bands, bullae, multifocal subsegmental consolidation, random small nodules, focal ground-glass, and multifocal ground-glass [8].
Pulmonary lesions analyses were independently performed by two experienced thoracic radiologists and the disagreeing cases were resolved through a posteriori consensus.
The number of lesions found on HRCT was quantified in each patient, i.e., for each patient was added the number of lesions regardless the degree found.
Mann-Whitney test was used to statistically analyze the number of lesions found on HRCT. A p d" 0.05 was considered statistically significant.
The study was approved by the local Institutional Review Board and Ethics Committee and a written postinformed consent was obtained from the child's legal representative, under the protocol number 3727/2006.
RESULTS
In the whole group, the median lesion per patient found on HRCT was seven lesions. Three patients presented normal CTs, two in Group A and one in Group B.
In Group A, the most frequent finding was multifocal ground-glass affecting three patients. Atelectases were not found. Figure 1 shows the tomographic findings in this group. In Group B, multifocal ground-glass lesions, atelectasis and areas of low attenuation and reduction in vessel caliber were preponderant in five patients (62.5%); two (25%) presented parenchymal bands, multifocal subsegmental consolidation and focal ground-glass, and one (12.5%) presented bullae.

Fig. 1 - Percentage of tomographic findings in patients pharmacologically treated for closure of the ductus arteriosus (Group A)
Figure 2 shows the tomographic findings of Group B in patients who evolved with pharmacological treatment failure and later underwent surgical treatment, and I n those who underwent surgical treatment alone.

Fig. 2 - Percentage of tomographic findings in patients surgically treated for closure of the ductus arteriosus (Group B)
The most frequent found lesions in both groups were multifocal ground-glass, atelectasis, and areas of low attenuation and reduction in vessel caliber as depicted in Figures 3 to 5.
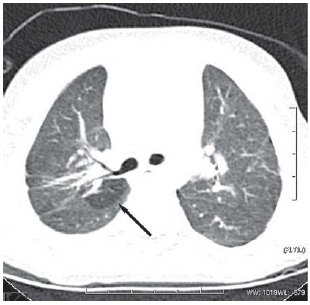
Fig. 3 - M.N.R., female, 2 years and 9 month-old, birth weight of 640 g. Image obtained in the level of main bronchi. Hypoattenuating subsegmental lesion of well-defined contours with area of low attenuation and reduction in vessel caliber aspects in the superior segment of inferior lobe of right lung (arrows)
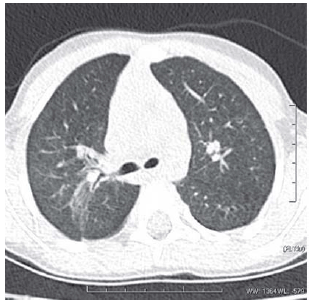
Fig. 4 - R.A.M, male, 4 years and 9 month-old, birth weight of 980 g. Image obtained in the pulmonary superior filed with a window for lung parenchyma. Presence of multifocal ground-glass opacity in the superior lobe of right lung (arrows)
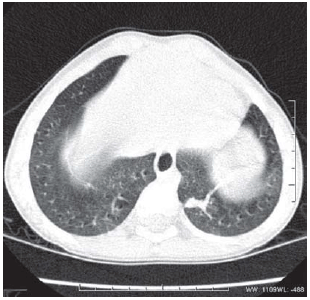
Fig. 5 - E.R.S., case 2, male, 3 years old and birth weight of 480 g. Image obtained from the bases of lungs. Hypoattenuating lesion in band in the superior segment of inferior lobe of left lung with subsegmental atelectasis aspect (arrows)
Comparing statistical outcomes through the application of Mann-Whitney test, patients who were pharmacologically treated only (Group A) vs those who in some time underwent surgical treatment (Group B), we get subsides to evaluate evidences related to the statistical significance of the difference between the medians obtained for the following variables: intubation time, duration of oxygen dependence, and hospitalization, which seemed to be statistically significant, as shown in Table 2.
These findings indicated that patients undergoing surgical closure of the ductus arteriosus (Group B) had the following the higher averages scores: intubation time, duration of oxygen dependence, and hospitalization.
In the dispersion plot (Figure 6), correlating the number of lesions and the type of treatment employed between surgical (Group B) and pharmacological (Group B) patients, it is observed a higher number of lesions in those who underwent surgery. There was statistical difference (p=0.0787).

Fig. 6 - Individual dispersion values plot relative to the number of lesions, according to surgical (Group B) and pharmacological treatment (Group B) for closure of the ductus arteriosus
With the advance of neonatal assistance, survival, PDA and BPD have significantly increase in the premature infants. In those with an extremely low birth weight, the outcomes were highly adverse influencing the cognitive development and the pulmonary function [9].
Several factors can affect the immature lung and develop BPD, such as intubation time, oxygen exposition time, infection, and presence of PDA [10]. As we can observe in Table 1, the mean intubation time was 34±18.4 days and the mean oxygen dependence was 77±24.5 days, which supports the pulmonary insult severity the premature patients suffered in the neonatal period.
The closure of patent ductus arteriosus with hemodynamic repercussion in premature infants should be performed as earlier as possible, whether by pharmacological or surgical intervention in order to try reducing morbidities. Although, a multicenter study does not indicate which of the two therapies should be the first choice in newborn infants [10], Méier et al. [11] showed that premature newborns with respiratory distress syndrome, who have been administered prostaglandin when underwent surgery as a result of this treatment failure, have the worst outcome in comparison to those who underwent surgery as a first therapy choice.
Pharmacological treatment is undertaken administering oral inhibitors of prostaglandin synthesis, among which are indomethacin, and more recently ibuprofen, besides supportive measures, such as fluid intake restriction and diuretics use [12,13].
Indomethacin is the drug of choice in the majority of neonatology centers and it was administered to our patients. The constrictor therapy effect with indomethacin combined with fluid intake restriction and therapy with diuretics can explain the development of intestinal ischemia and organ dysfunction [7]. Other implications observed are trend to bleeding due to platelet dysfunction, necrotizing enterocolitis (NEC), renal function impairment with diminished urine output, resulting in overhydration with reduced pulmonary compliance and increased ventilation support time and transient heart dysfunction [5,12,14]. Such a fact justified the referral of three children directly to surgical treatment because one already presented intracranial hemorrhage and the other two increased serum creatinine.
In a study carried out with 21 premature infants with PDA, it was observed the closure of patent ductus arteriosus using indomethacin in 19 (90.4%) patients and the re-opening in 6 (31.5%) [14]. The data are not compatible with our sample, once 11 (78.6%) patients who underwent indomethacin treatment, five (45.4%) presented PDA re-opening, what can be explained by PDA association with infection. This was not a studied variable; however, all the patients developed infection and required antibiotics during hospitalization.
On the strength of the above-mentioned, in another study comparing premature infants with or without infection, the authors observed a significantly higher failure rate with indomethacin - 68% in infected children and 17% in noninfected ones - regardless birth weight, age, the route used to administer indomethacin (orally or intravenously), and the dose administered [9].
The re-opening rate of ductus arteriosus with pharmacological treatment is high in premature infants with less than 1000 g and lower in those over 1500 g. The success in closure of the patent ductus arteriosus is rather related to both birth weight and gestational age than to the indomethacin plasma concentration peak [13,16]. The median birth weight in both groups, surgical and pharmacological, were 840 g and 1055 g, respectively. This confirms that premature infants with less than 1000 g are more likely to have pharmacological treatment failure.
Surgical ligation is usually reserved either for PDA refractory to pharmacological treatment or for the impossibility to perform this treatment. Due to its low morbidity rate, it has been proposed as a primary treatment for PDA in some centers by its higher efficacy and lower morbimortality [14]. However, this remains a controversial procedure, and a more recent study has reported that PDA ligation is largely performed in premature infants, despite the clear evidence of a better outcome regarding pharmacological treatment [13].
In a randomized study comparing surgical vs pharmacological treatment with indomethacin in premature infants with less than 1750 g, there was no significant difference in days of hospitalization, lung lesions, necrotizing enterocolitis, and intracranial hemorrhage [13]. Although, our study was not carried out in a similar fashion, we did not find significant differences as for the number of lung lesions between both treatments; however, in the surgical group there has been found differences in intubation, oxygen dependence, and hospitalization mean times. This can be explained because previous unsuccessful treatment with indomethacin could have delayed surgical intervention and influenced the morbidities. Furthermore, pharmacological treatment was contraindicated to the patient who underwent direct surgical treatment, what could have selected the more critically patients to this group.
HRCT allows a yet more detailed evaluation of the lung parenchyma, by analyzing the distribution of morphofunctional changes throughout the bronchi, lymphatic routes, and inside the secondary lobule [16]. Its use is still limited, despite both sensitivity and specificity in comparison to thoracic radiography, because the radiation dose is higher and its cost is expensive.
A study conducted with 62 extremely low birth weight (ELBW) premature infants, close to their discharge from hospital, showed that most of infants presented more than one alteration on HRCT, described as ground-glass opacity, parenchymal bands, atelectasis, and bullae [17], which are very close to what we have found in our study, once the areas of low attenuation, parenchymal bands, multifocal segmental consolidation, and focal and multifocal ground-glass were present in children treated both surgically and pharmacologically.
Atelectasis, a complication sometimes resulting from surgical treatment drew our attention, even though not statistically significant, because these complications were found in patients who underwent surgical treatment only, suggesting that maybe the surgical aggression could provoke this late consequence in some patients, and that future studies need to be undertaken addressing this significant concern.
On the other hand, of the three patients who presented normal HRCT, two were treated pharmacologically and one surgically only, suggesting that regardless the therapy chosen to prevent late pulmonary outcomes, the bottom line is that early closure of the ductus arteriosus may be achieved pharmacologically using or by surgery.
It is important to emphasize that the goal of our study was to verify lung lesions and correlate them with the treatment for PDA closure. Evaluation of respiratory sign and symptoms was not included in our inclusion criteria, so that there was not any correlation between radiological and clinical image. However, it was naturally observed that the children surgically treated, in consequence of their critically severe health conditions and because most often they have undergone pharmacological treatment previously, presented much more lung infections and, consequently, much more frequent hospitalizations. This fact has drawn our attention and may be eventually addressed in another study.
CONCLUSION
Lately performed high-resolution computed tomography has shown no significant difference between either pharmacological or surgical treatment aiming at the closure of the ductus arteriosus in parenchymal lesions in premature infants with PDA who evolved to bronchopulmonary dysplasia.
REFERENCES
1. Monte LFV, Silva Filho LVF, Miyoshi MH, Rozov T. Displasia broncopulmonar. J Pediatr. 2005;81(2):99-110.
2. Azevedo ABC, Guimarães SMM, Tavares Jr WC, Calderato D, Leão Filho M, Ferreira CS, et al. Avaliação da tomografia de alta resolução versus radiografia de tórax na doença intersticial pulmonar na esclerose sistêmica. Radiol Bras. 2005;38(2):95-9.
3. Pereira-Silva JL, Kavakama J, Terra Filho M, Porto NS, Souza Jr AS, Marchiori E, et al. Brazilian Consensus on terminology used to describe computed tomography of the chest. J Bras Pneumol. 2005;31(2):149-56.
4. Santos MLO, Marchiori E, Vianna AD, Souza Jr AS, Moraes HP. Opacidades em vidro fosco nas doenças pulmonares difusas: correlação da tomografia computadorizada de alta resolução com a anatomopatologia. Radiol Bras. 2003;36(6):329-38.
5. Ellison R, Peckham G, Lang P, Talner NS, Lerer TJ, Lin L, Dooley KJ, et al. Evaluation of the preterm infant for patent ductus arteriosus. Pediatrics. 1983;71(3):364-72. [
MedLine]
6. Laughon MM, Simmons MA, Bose CL. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr. 2004;16(2):146-51. [
MedLine]
7. Herrera C, Holberton J, Davis P. Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants (Cochrane Review). In: The Cochrane Library, Issue 3, 2005. Chichester, UK:Jon Wiley & Sons;2005.
8. Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113(4):781-9. [
MedLine]
9. Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996;128(4):470-8. [
MedLine]
10. Malviya M, Ohlsson A, Shah S. Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants (Cochrane Review). In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons; 2005.
11.Méier MA, Jazbik WC, Jazbik JH, Oliveira JC, Aldrovando J, Silva JC, et al. Manuseio do canal arterial patente no prematuro com síndrome de angústia respiratória: ligadura ou indometacina? Rev Bras Cir Cardiovasc. 1989;4(1):9-20.
12. Flores M. Ibuprofen: alternative treatment for patent ductus arteriosus. Neonatal Netw. 2003;22(2):27-31. [
MedLine]
13. Brooks JM, Travadi JN, Patole SK, Doherty DA, Simmer K. Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F235-9. [
MedLine]
14. Malviya M, Ohlsson A, Shah S. Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003;(3): CD003951. [
MedLine]
15. Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2003;(2):CD003481. [
MedLine]
16. Rossi UG, Owens CM. The radiology of chronic lung disease in children. Arch Dis Child. 2005;90(6):601-7. [
MedLine]
17. Mello RR, Dutra MV, Ramos RJ, Daltro P, Boechat M, Lopes JMA. Lung mechanics and high-resolution computed tomography of the chest in very low birth weight premature infants. São Paulo Med J. 2003;121(4):167-72. [
MedLine]








 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license