INTRODUCTION
The concept of rapid preparation of the left ventricular (LV) was introduced by the group from Boston in 1989 to treat transposition of the great arteries (TGA) after the neonatal period. Retraining of the subpulmonary ventricle was obtained in a mean time of nine days and after this Jatene's operation was performed [1]. These authors achieved good preliminary results, however, they were not reproduced in other centers due to high morbimortality. One of the greatest limitations of this technical approach is related to the lack of adjustability of the pulmonary trunk (PT) banding. The degree of PT banding may be inadequate or imprecise and can cause an important acute systolic overload of the LV [2, 3].
Heart hypertrophy is the main adaptive response of a heart submitted to physiological or pathological overload. It is interesting to note that the acquisition of LV mass during physical conditioning of athletes who practice swimming for example, reaches a peak in about only one week of training. Then the LV mass remains relatively constant [4]
In an attempt to improve the rapid hypertrophic process without causing injury to the myocardium of the subpulmonary ventricle, we tried to find an analogy between the physiological hypertrophic processes in static-type exercises and intermittent PT banding, where periods of systolic overload are alternated with periods of subpulmonary ventricle relaxation.
We formed a hypothesis that the subpulmonary ventricle, submitted to the gradual and progressive systolic overload alternated with periods of relaxation, could cause a more beneficial hypertrophic process, similar to the process observed in the myocardium of athletes who perform exercises causing systolic overload. Faced with the possibility of intermittently adjusting the systolic overload of the subpulmonary ventricle, a better understanding of the changes occurring during this acute hypertrophy process is necessary with the objective of evaluating its impact on myocardial function. Thus, induction of a more physiological hypertrophy may be achieved, in order to preserve the future ventricular function.
The objective of this study was to compare acute myocardial hypertrophy of the subpulmonary ventricle (RV) of two groups of young goats, with the first one being submitted to continuous and progressive RV systolic overload and the second undergoing intermittent RV systolic overload using an adjustable PT banding device. We also analysed and compared rapid hypertrophy, from hemodynamic and echocardiographic points of view, in the two processes of systolic overload of the subpulmonary ventricle (intermittent versus continuous). Moreover the weight and myocardial water content were compared between the study groups and a control group.
METHOD
This study was approved by the Ethics Research Commissions of the Hospital das Clínicas and the Medical School of the University of São Paulo in accordance with the norms on animal use in teaching and research established in the "Guide for the Care and Use of Laboratory Animals" (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, D.C., 1996) and the ethical principles for animal experimentation of the Brazilian College of Animal Experimentation (COBEA).
Twenty-one goats with ages between 30 and 60 days were included in the study and divided into three groups: Control (n = 7; weight = 7.5 ± l.9 kg, no surgical procedure), Continuous (n = 7; weight = 9.3 ± 1.4 kg, continuous systolic overload of the RV), Intermittent (n = 7; weight 8.1 ± 0.8 kg, 12 hours/day of intermittent systolic overload of the RV), all studied for a period of 96 hours.
Preoperative assessment
All the animals were submitted to echocardiography (Apogee CX, Advanced Technology Laboratories, Bothell, WA, USA), in the preoperative period to confirm the thickness of the RV free wall in relation to the left ventricle (LV).
Anesthesia
The animals remained 24 hours fasting before the surgery. Anesthetic induction was achieved with ketamine (30 mg/kg, intramuscular). The animals were weighted and subsequently their jugular veins were punctured with a Jelco nº18 catheter for the administration of drugs and infusion of saline solution. The animals were then sedated with Nembutal (from 5 to 10 mg/kg, endovenous) and intubated. Mechanical ventilation (Harvard 708, South Natick, MA, USA) was established with an inspired oxygen fraction of 100% and tidal volume of 15 mL/kg. The animals were positioned in the right lateral decubitus position, monitored by ECG and prepared for the sterile procedure. Anesthesia was maintained with sodium pentobarbital (Nembutal, 5 -10 mg/kg, intravenously) and ketamine (1 mg/kg endovenously). All animals received antibiotic therapy initiated before the surgery and maintained until the end of the protocol (cephazolin 500 mg and gentamicin 10 mg, intramuscular, every 12 hours). Digoxin (0.005 mg/kg endovenously every 12 hours) and heparin (2500 IU every 12 hours subcutaneously) were also administrated until the end of the protocol.
Surgical procedure
Left lateral thoracotomy was performed at the 4th intercostal space. The lung was dislocated and the pericardial sac was opened. An adequate view of the RV outflow tract, the PT and descending thoracic aorta were obtained. At this moment, three previously-heparinized catheters (intracath 17G) were implanted in the RV, PT and descending thoracic aorta. The catheters were fixed with prolene 5-0 purse-string sutures and exteriorized through the thoracic wall, near to the spinal column, where they were also anchored to the skin using 3-0 cotton thread. Subsequently, these catheters were tested (permeability and pressure curves) and maintained heparinized. Pressures, proximal and distal to the adjustable PT banding device, were measured as was the systemic arterial pressure using the ACQ Knowledge 3.01 software system (Biopac Systems, Inc., Goleta, CA, USA).
PT banding device
The PT was dissected to implant the PT banding device as described and utilized in previous publications [5, 6]. The cuff occluder was immediately positioned over the pulmonary valve and fixed to the adventitial layer of the PT to prevent its migration. The insufflation button of the device was implanted subcutaneously thereby allowing fine percutaneous adjustment of the diameter of the banding ring.
After device implantation, the thorax was drained. The ribs and thoracic wall tissues were closed by layers. After approximately six postoperative hours, the thorax drain was removed after verifying minimal drainage, the absence of broncopleural fistulae and good pulmonary expansion.
Protocol of RV systolic overload
RV training was started after total recovery from the surgery (72 hours of convalescence). With the animal conscious and immobilized on a special stretcher, the RV, PT and aorta basal pressures were measured with the PT banding device completely deflated. After measurement of the basal pressures, percutaneous insufflation of the device with saline solution was initiated using an insulin syringe, observing the pressures curves of the RV and aorta with an aim of reaching a RV pressure of approximately 70% of the systemic systolic pressure, as long as this did not drop by 10% or more.
If the animal presented clinical signs of significant hypoxia, such as agitation, breathing difficulties or arrhythmias, the volume of the device was reduced to a tolerable level. Insufflation of the device and measurement of the aortic, RV and PT pressures were performed every day for the two study groups. The volume of fluid in the device was measured and compared to the previous day to assess possible losses. The device was insufflated again, trying to achieve the desired parameters; in general it was possible to increase the volume every day. The pressure gradient between the RV and the pulmonary arterial trunk was calculated by subtraction of their systolic pressures.
Training of the continuous group
The animals remained under continuous systolic RV overload for a period of 96 hours with progressive insufflations at 24-hour intervals to the maximum tolerated limit.
Training of the intermittent group
The animals were submitted to four 12-hour periods of RV systolic overload (during the day), alternated with 12 hours of relaxation (at night), over the same period of 96 hours as the continuous group. The pressures were measured both during the day and at night.
Echocardiographic study
All animals were submitted to echocardiographic assessment by the same specialist, before the start of the insufflation protocol and on each day after the initial insufflation of the device, to assess the RV hypertrophy process of both study groups over the 96 hours of RV systolic overload. The animals of the Control Group were evaluated just once before being sacrificed.
Transducers of 7.5 MHz were utilized to obtain the images and 2.5-MHz transducers to assess blood flows. The thickness of interventricular septum and LV posterior wall were measured at M-mode at the end of diastole by a transversal parasternal plane at the papillary muscles. The RV/PT pressure gradient caused by the device was obtained by continuous Doppler. The RV free wall perimeter and its thickness were measured by 2-dimensional echocardiogram always at the end of diastole using the same parasternal plane near to the great vessels and the papillary muscles. The measurements of the RV perimeter were indexed to the RV wall thickness at the site of the perimeter measurement. These measurements were also taken for the four-chamber longitudinal plane. The RV free wall thickness is the mean of three measurements taken in each examination. The right ventricle final systolic (FSV) and final diastolic volumes (FDV) were obtained by the formula area x length in the longitudinal four-chamber slice. The hemodynamic efficiency was evaluated from the RV ejection fraction (RVEF), which was attained from the difference of the volumes using the formula proposed by Pontes et al. [7].
RVEF= (FDV - FSV) x FDV-1
Weighing the heart masses
After the end of the protocol of each animal, sacrifice was achieved by resection of the heart. Before anesthetic induction, the RV, PT and aorta pressures were measured. After general anesthesia using ketamine (30 mg/kg, IM) with Nembutal (15mg/kg, EV) and orotracheal intubation, left thoracotomy was performed at the site of the previous incision to expose of heart. Dissection of the superior and inferior vena cavas and great arteries were performed. After increasing the anesthesia, heparin (50 mg EV) and potassium chloride were administrated to cause cardiac arrest.
The heart was then resected. The epicardial fat was carefully removed and the ventricular and septal walls separated according to the technique published by Fulton et al. [8]. The aorta and PT were resected near the semilunar valves. Atria were resected together with the atrioventricular valves and identified. The RV free wall was separated from the interventricular septum, incising it in the same direction as the anterior and posterior interventricular arteries. The same procedure was performed to separate the LV free wall from the septal wall.
Subsequently, the RV, LV, interventricular septum and atria were weighed using a digital balance (Mettler AE-200, Mettler-Toledo AG, Greifensee, Switzerland). Due to the variation in the weight of animals, the measurements were standardized by indexing the weight of the heart muscle mass to respective body weights of the animals. The indexed weights are expressed in g/kg. The animals of the Control Group were also submitted to the same scarification, resection and weighing procedures.
Tissue water content
The next step was to select samples of each of the cardiac walls to assess water content (WC). The initial weight (IW) of each sample was obtained. The samples were then placed in appropriate flasks, closed with filter paper and duly identified, before being placed in an incubator (temperature: 55-60º C). After about 70 hours of dehydration, each sample was again weighed to obtained the dry weight (DW). The percentage water content was then obtained by the following formula, assuming that the distribution of water was homogenous in the septum and in the ventricles:
WC (%) = 100 - (DW x IW-1 x 100)
The water content of the heart muscles in the Continuous and Intermittent Groups were compared to the Control Group to check if the increase in weight of the RV in the study groups was associated to myocardial edema.
Statistical analysis
The normality of the distribution of each variable was assessed by means of the Kolmogorov-Smirnov test. Comparisons of the medians of variables such as the RV/LV ratio and RV heart muscle thickness were evaluated using the Kruskal-Wallis non-parametrical test followed by the Student-Newman-Keuls multiple comparison test. The comparisons of the aortic systolic pressures, RV ejection fraction (measured by echocardiography), RV/PT pressure gradient, perimeter and RV final diastolic volume in the Continuous and Intermittent Groups were made by analysis of 2-way variance (ANOVA), followed by Fisher's exact test for multiple comparisons. However, the masses of the RV, LV and septum and the water content of the RV, LV and septum were compared using the one-way ANOVA test followed by Bonferroni's test for multiple comparisons. The systolic overload applied to the RV of the Continuous and Intermittent Groups was evaluated by calculating the areas under the curves (trapezoidal method), which describe the behavior of the pressure gradient between the RV and the PT (RV/PT) in these groups. A comparison of the areas under the curves was made by means of Student's t-test for unmatched data. Values are presented as means ± standard deviation (SD) for analyses using parametrical tests and as medians with respective interquartiles (25%-75%) using non-parametric tests. In all cases, the level of significance employed was 5%. Statistical analyses were made with the GraphPad Prism v.4 (San Diego, CA -USA) and Statistics v.6 software programs (Tulsa, OK - USA).
RESULTS
All animals completed the 96-hour protocol of RV systolic overload. The weight of the animals of the three groups were similar to each other (p=0.11). The Intermittent Group supported a higher filling volume of the inflatable occluder than the Continuous Group (0.63 mL ± 0.19 mL versus 0.47 mL ± 0.16 mL, p= 0.001). There was a minimal loss of volume injected into the device during the study period.
Hemodynamical measurements
Systemic arterial pressure
The systemic systolic pressure of the two study groups of animals was analysed by the two-way ANOVA parametric test, demonstrating that there was no significant difference using the different overload stimuli (p=0.21 and p=0.12 for group and time, respectively), independent of the method (continuous or intermittent). It is interesting to observe that there was a trend of lower systolic pressures in the Continuous Group when compared to the Intermittent Group.
RV/PT pressure gradient
The two-way ANOVA test demonstrated a statistical significance (p=0.02) for the RV/PT gradient ratios. In respect to RV training, Figure 1 demonstrates a progressive increase in pressure gradient for RV/PT only for the Intermittent Group at 48 hours (72.00 mmHg ± 15.17 mmHg) and 72 hours (80.00 mmHg ± 12.99 mmHg) when compared to the basal measurements (9.57 mmHg ± 9.45 mmHg; p< 0.05). However, there was no significant difference in the values of gradient caused by the RV between the Continuous and Intermittent Groups at all time intervals (0, 24, 48, 72 and 96 hours, p> 0.05).
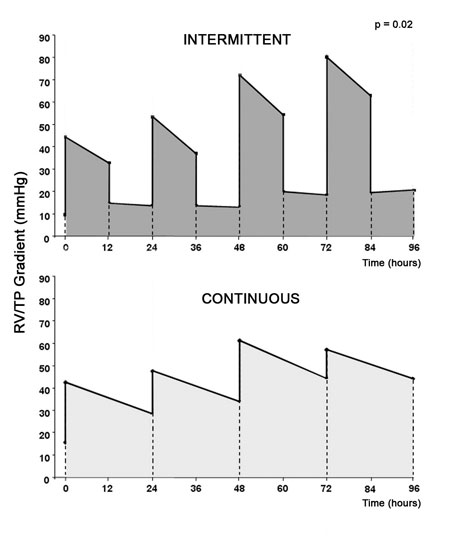
Fig. 1 - Diagram of RV systolic overload. Upper graph: RV/PT gradient (mmHg) of the group submitted to 12 hours of RV systolic overload alternated with 12 hours of relaxation. Lower graph: RV/PT gradient (mmHg) of group submitted to continuous RV systolic overload. (* p=0.02)
The drop in the RV/PT gradient at the last measurement (96 hours) of the Intermittent Group is due to the fact that the animals of this group were relaxing. In spite of this, higher gradients caused by the RV at this moment were observed compared with the basal gradient, even with the adjustable banding device deflated. However, these differences were not statistically significant. Figure 2 shows the systolic overload area applied to the RV in the two groups. The data were obtained by calculating the product of the pressure gradient RV/PT over time of systolic overload. The systolic overload area was lower in the Intermittent Group when compared to the Continuous Group (p=0.002).

Fig. 2 - Diagram of RV systolic overload area. Overload area of groups submitted to continuous banding versus intermittent PT banding. (*P=0.002)
The RV/LV pressure ratios of both groups of RV systolic overload are demonstrated in Table 1. Together with the RV/PT gradient, we observed a progressive increase of the RV/LV ratio in the two groups when compared to the basal values (p<0.05). Nevertheless, higher values were observed in the Intermittent Group when compared to the Continuous Group, at 24, 48 and 72 hours. The significant drop in the RV/LV ratio of the Continuous Group at 96 hours of protocol (p<0.05) may be related to the gradual loss of volume from the adjustable PT banding device. In regards to the Intermittent Group, as the device was deflated during the 96-hour hemodynamics measurements, due to the prior relaxing period a lower RV/LV ratio was expected, however it was still higher than the basal value (p<0.05).
Echocardiographic findings
Heart wall thicknesses
All animals included in the protocol presented with thinner RV thicknesses compared to the septum and the LV at the start of the study. The Kruskal-Wallis' non-parametrical test demonstrated that there was no alteration in the LV thickness or of the septum with the continuous or intermittent overloads (p=1.00).
Figure 3 shows a progressive increase in the RV wall thickness during the protocol. Both groups presented with significant increases in the RV free wall thickness. Nevertheless, this increase was significantly greater in the Intermittent Group with an increase of 132.1% at the end of the protocol compared to an increase of 63.7% in the Continuous Group (p<0.05). The LV contractility was preserved during the protocol.
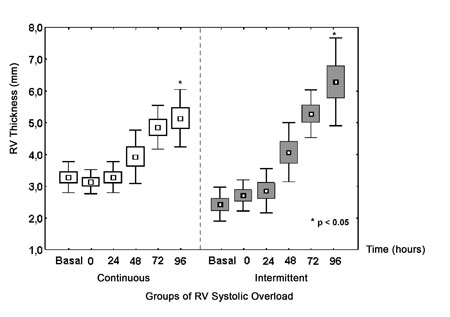
Fig. 3 - Echocardiographic measurements of RV. RV free wall thickness of groups submitted to continuous and intermittent systolic overload (*p<0.05)
The two-way ANOVA test gave a p-value < 0.001 for the RV/PT pressure gradients. However, the multiple-comparison test did not show any statistical difference between the groups at any time interval (0, 24, 48, 72 and 96 hours). Similar to the measurements attained using catheters, a significant increase in the RV/PT gradient of the Intermittent Group was noted 72 hours after the start of the systolic overload (30.29 mmHg ± 6.16 mmHg) in relation to the basal measurement (2.14 mmHg ± 1.46 mmHg; p<0.05) by echocardiography. Different to the measurements observed using catheters, the Continuous Group demonstrated increments in the RV/PT gradient greater than the basal value after 48, 72 and 96 hours of systolic overload as identified by Doppler (p<0.05).
It is important to highlight that according to what we observed by hemodynamic measurements verified by catheter, at 96 hours of RV overload in the Intermittent Group, with the RV already trained, higher RV/PT pressure gradients than the basal measurements were also observed despite of the animals being in a period of relaxation. However, these differences were not statistically significant.
RV end diastolic volume
Figure 4 demonstrates the percentage variation of the RV diastolic volume during the systolic overload protocol compared to the preoperative echocardiographic evaluation. ANOVA of the RV end diastolic volume showed a significative difference between the groups (p=0.01). An analysis of multiple comparisons (Fisher's test) revealed a greater dilatation of the RV in the Continuous Group after 24 hours of systolic overload when compared to the basal reading and greater dilations at the 48-, 72- and 96-hour time intervals of the Intermittent Group (p<0.03). Temporal differences in variations of the RV end diastolic volume within the groups were not observed during the study (p=0.24).

Fig. 4 - RV diastolic volume variation. Percentage variation of RV end diastolic volume of groups submitted to continuous systolic overload versus intermittent RV systolic overload. (p=0.01)
The RV ejection fractions of the two groups were normal during the RV systolic overload protocol, thus, no harm was caused by adjusting the PT band. ANOVA did not identify significant changes of this parameter between the groups (p=0.07), although lower increments of the RV ejection fraction were seen in the Continuous Group during the protocol compared to the Intermittent Group.
RV perimeter
Table 2 illustrates the RV perimeters measured near to the great vessels and indexed to the RV wall thickness for the two study groups during the protocol. ANOVA demonstrated a significative difference in relation to time (p=0.006), but no difference between the groups (p=0.18). In the multiple comparisons (Fisher's test), a smaller RV perimeter was observed in the Intermittent Group at 96 hours of training compared to the first day of training of the Continuous Group (p<0.05). In respect to the percentage variation of the RV perimeter in comparison to the preoperative measurements, although the Continuous Group always had positive values and values similar to the Intermittent Group, no statistically significant differences were evidenced (p=0.18).
Weighing the heart tissue
Table 3 shows the heart tissue weights indexed to the animals' body weight. One-way ANOVA demonstrated that there were only significant differences in the RV (p=0.001) and septum masses (p=0.026). The RV hypertrophic process did not influence the LV muscle mass (p=0.53). The systolic overload determined similar increases in the RV masses of both groups compared to the Control Group, with 55.6% increase in the Continuous Group and 88.9% in the Intermittent Group. In respect to the increase in septal muscle mass, a significant increase of 40% was observed in the Intermittent Group when compared to the Control Group (p=0.03).
When the RV mass was indexed to the systolic overload area (Figure 5) for the Continuous and Intermittent Groups, a more significant increase was observed in the Intermittent Group. This data demonstrated that, for similar variations of systolic overload applied to the RV, the increase in mass was greater in Intermittent Group (p=0.02).
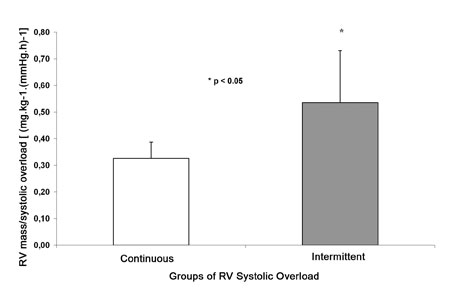
Fig. 5 - RV mass Index. RV mass indexed by area of systolic overload of continuous and intermittent groups ( *p<0.05)
In spite of RV and septum weight gains in the study groups, there were no significant differences in the RV myocardial water content between these groups and the Control Group according to one-way ANOVA (RV: p=0.10; septum: p=0.45 and LV: p=0.88).
Discussion
Percutaneous adjustment of PT banding is an extraordinary instrument to manage the type and quality of systolic overload applied to the subpulmonary ventricular myocardium. A definition of the ideal frequency and amount of overload required to cause physiological cardiac re-hypertrophy has not yet been established.
This study tries to assess and improve the re-hypertrophy process of the subpulmonary ventricle, attempting to find alternatives to improve the quality of the myocardial hypertrophy by studying concepts from physical training programs of the athlete's skeletal striated musculature.
Training protocol
It is very clear in this study that, in spite of there being no harm nor differences in RV performance between the two study groups resulting from the systolic overload imposed by adjustable PT banding, the RV training program to promote the myocardial hypertrophic process was more efficient in the Intermittent Group than in the Continuous Group. A proportionally smaller overload in the Intermittent Group caused significantly greater hypertrophy, as was seen by the RV free wall thickness at echocardiography and by greater increases in septal mass.
The group of Perrino [9] from Duke University, USA, analysed the development of LV hypertrophy in rats submitted to continuous and intermittent aortic arch banding, comparing them to two groups of rats submitted to running and swimming exercises during a period of four weeks. Both physical exercises and intermittent overload were performed twice per day, during a 90-minute period. The authors observed an increase in the LV weight of the intermittent group similar to the Running and Swimming Groups, in contrast to the group submitted to the continuous LV overload, which presented a more significant hypertrophic response. In spite of the greater hypertrophy in the Continuous Group, there was significative deterioration in the heart function during the study protocol, as demonstrated by echocardiography.
Undoubtedly, the current study would better show the trend of worse hemodynamic performance of the Continuous Group, if it were over a period of more than four weeks as in the study of Perrino et al. [9].
Echocardiographic parameters
The echocardiographic findings indicate a significative increase in the free wall thickness of both study group subpulmonary ventricles, corroborating with the increase in the RV tissue weight. However, the septal thicknesses did not change according to echocardiographic analysis, different to the significant increase of the septal mass in the Intermittent Group. Perhaps, this divergence may be explained by a higher protein content and muscle density of this wall, but without a proportional macroscopic increase visible by echocardiography. Additionally, a greater RV dilatation and a trend of greater increase in the RV perimeter observed by echocardiography in the animals of the Continuous Group may represent a greater physiopathological effect on the myocardium submitted to continuous systolic stress without alternating relaxation.
Carrol et al. [10] found intense inflammatory infiltration in the myocardium of swine submitted to pulmonary banding after 7 hours of systolic overload. Areas of variable degrees of cellular necrosis in the hypertrophied myocardium [11-13] and a consequent late ventricular dysfunction [14] have also been demonstrated in hearts submitted to acute systolic stress, probably due to imbalance between the oxygen supply and demand ratio in the hypertrophic myocardium.
LV hypertrophy induced by systemic arterial hypertension results in a reduction of the coronary flow reserve (number of arterial vessels/unit volume) [15]. In studies on RV hypertrophy, a coronary arterial tree remodeling has already been seen with an increased number of vessels and a reduction in the resistance to intracoronary flow [16]. However, it is still not clear if this difference occurs due to hypertension imposed on the coronary arteries in LV hypertrophic models. However, it is interesting to observe that, in hearts with TGA, the LV is connected to the pulmonary artery, and thus, pulmonary banding does not cause hypertension of the coronary system, a fact that is not assessed in experimental studies, due to the difficulty of creating a similar model. It may be that this detail is important in the understanding of the phenomena that occurs with the induced hypertrophy of these hearts.
Apart from possible cellular necrosis, there is a change in the genetical profile of cardiomyocytes with proteic alterations that are related to worsening of myocardial contractility.
Maybe intermittent periods of relaxation during the protocol may optimize subendocardial coronary flow and, consequently, provide more substrates to the myocardial hypertrophic process, thereby limiting the degree of continuous systolic stress imposed on the RV of the Continuous Group.
Weighing the heart tissues
The greater efficiency of intermittent systolic overload may be related to the triggering of the hypertrophic stimulus and the proteic synthesis cascade in the same manner as in the Continuous Group, but with less energy loss of the myocardium. Probably, the mechanism of this hypertrophic process triggered by the molecular cascade may be developed in good conditions during periods of relaxation and with ideal oxygen transport and, hence, without the development of fibrosis resulting from relative ischemia.
This hypothesis is corroborated by the studies of Le Bret et al. [17] who caused RV hypertrophy of sheep in only two hours daily of RV systolic overload during a period of five weeks. Fibrosis was then observed in animals submitted to a conventional banding regime and in those submitted to only two readjustments of RV systolic overload during the five-week protocol.
Recently, several studies have recommended stem cell implantation in the myocardium, as a therapeutic alternative in the treatment of heart failure, with the goal of improving the ventricular function performance. The French group of Borenstein [18] reported in 2005, the first experimental study on cell cardiomyoplasty in a training protocol with PT banding, for possible clinical application in Jatene's two-stage operation. However, the authors did not observe additional hemodynamic benefits of this strategy in a group submitted to banding associated to cell transplantation in the subpulmonary ventricle.
Water content
This study demonstrated similar increases in RV mass in both study groups when compared to the Control Group. A greater increase of septal muscle mass was observed in the Intermittent Group in comparison to the Control Group. Probably, this increase in heart tissue weight was a result of increased proteic synthesis, as there was no significant difference in the RV myocardial and septal water contents between the study groups and the Control Group.
Implications
This 96-hour protocol analyzes the performance of acute RV hypertrophy of young goats submitted to two programs of systolic overload, demonstrating the greater efficiency of the Intermittent Group. In patients with TGA after the neonatal period or in those already submitted to the atrial repair, such as the Senning or Mustard procedures, and even in patients with repaired TGA, who evolve with right ventricular dysfunction (systemic), the left ventricle morphologically (subpulmonary) requires retraining to support the systemic circulation after Jatene's operation. To achieve this objective with conventional PT banding is problematic, as some patients need to be reoperated to relieve the banding due to the excessive constriction of the banding leading to ventricular dysfunction, or to tighten the banding in cases of inadequate left ventricle training, because of a loose band. These reoperations are associated to high morbimortality rates. Clinical application of an intermittent program of subpulmonary ventricle retraining in this setting could reduce the postoperative complications between the two-stages of surgical treatment. It is not know if the response to intermittent systolic overload stimulus in adult animals will be similar, considering the clinical application of Jatene's operation in older children and teenagers.
In the clinical experience of Mavroudis & Backer [19], the mean time between conventional banding and conversion of the atrial repair to the Jatene operation was 15.6 months in patients with ages between 2 to 23 years. These authors demonstrated that left ventricular dysfunction occurred after the banding, as was confirmed by transesophageal echocardiography performed in the operating room. Indeed, the physiopathology involved in this procedure is the same as what occurs in LV rapid training, as pulmonary banding or systemic pressure will cause the same LV hypertrophic stimulus. LV dysfunction can occur over the long-term, probably related to the degree of abrupt systolic stress imposed on the ventricle during its retraining.
In the clinical experience of the Boston group, Boutin et al. [20] associated late left ventricular dysfunction to an extreme acute overload in patients submitted to Jatene's operation after LV rapid training. This dysfunction was inversely proportional to a quicker hypertrophy after PT banding.
Maybe our intermittent systolic overload model promotes a more physiological subpulmonary ventricular hypertrophy when compared to continuous banding, where the systolic stress is constant. With the proposal of a more efficient process, with intermittent overload in this setting, for the two-stage surgical treatment, it is possible to reduce the interval between the two operations and so reduce postoperative morbidity in preparing the left ventricle morphologically.
Future studies utilizing molecular biology as an instrument to analyze pathologic hypertrophic markers might compare the different training methods for the subpulmonary ventricle. As the pathologic overload stimulus is generally chronic while physiological overload is intermittent by nature, it is possible that qualitatively different overloads stimulate distinct hypertrophies in the heart, even if applied during similar periods of time, producing different phenotypic responses.
As the physiologic hypertrophy is characterized by normal or increased capillary density associated with little or no myocardial fibrosis, the morphological evaluation of suitable mechanisms of the contractile and non-contractile elements of the myocardium submitted to the acute hypertrophy process can also provide important contributions to the understanding of the fine adjustment in the preparation for Jatene's operation. The end point would be to minimize the cell lesions and maximize the efficiency of the pulmonary banding, thereby elucidating the best program of subpulmonary ventricular training.
CONCLUSIONS
Adjustable PT banding allows rapid RV hypertrophy in young goats, in groups submitted to a short period of continuous or intermittent RV systolic overload. The intermittent systolic overload allowed a more efficient hypertrophic process of the RV compared to the group submitted to continuous systolic overload, considering the greater septal hypertrophy. In spite of less systolic overload imposed on the RV of the Intermittent Group, the muscle mass acquired in this group was greater per unit of overload. There was no difference in the performance of the heart function between the two groups submitted to intermittent and continuous systolic overloads. The increase of mass identified in both study groups was probably due to an increased proteic synthesis and not to the accumulation of water in the heart tissues.
ACKNOWLEDGMENTS
Our thanks go to Dr. Gustavo José Justo da Silva, for his guidance with the statistical analysis and for his important suggestions which were included in this study.
REFERENCES
1. Jonas RA, Giglia TM, Sanders SP, Wernovsky G, Nadal-Ginard B, Mayer JE Jr, et al. Rapid two-stage arterial switch for transposition of the great arteries and intact ventricular septum beyond the neonatal period. Circulation. 1989;80(3 pt 1):I203-8.
2. Wernovsky G, Giglia TM, Jonas RA, Mone SM, Colan SD, Wessel DL. Course in the intensive care unit after 'preparatory' pulmonary artery banding and aortopulmonary shunt placement for transposition of the great arteries with low left ventricular pressure. Circulation. 1992;86(5 Suppl.):II133-9.
3. Takahashi Y, Nakano S, Shimazaki Y, Kadoba K, Taniguchi K, Sano T, et al. Echocardiographic comparison of postoperative left ventricular contractile state between one and two-stage arterial switch operation for simple transposition of the great arteries. Circulation. 1991;84(5 Suppl.):III180-6.
4. Ehsani AA, Hagberg JM, Hickson RC. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol. 1978;42(1):52-6.
5. Dias CA, Assad RS, Caneo LF, Abduch MCD, Aiello VD, Dias AR, et al. Modelo experimental de bandagem ajustável do tronco pulmonar para preparo rápido do ventrículo. Rev Bras Cir Cardiovasc. 2000;15(4):328-37.
6. Caneo LF, Dias CA, Assad RS, Abduch MCD, Aiello VD, Moreira LFP, et al. Preparo do ventrículo subpulmonar através de dois diferentes modelos ajustáveis de bandagem do tronco pulmonar: estudo experimental. Rev Bras Cir Cardiovasc. 2001;16(1):35-48.
7. Pontes SC Jr., Assef JE, Barretto RB, Chaccur P, Moreira DA, Nina VJS, et al. Estimation of right ventricular mass by two- dimensional echocardiography. J Am Soc Echocardiogr. 2005;18(5):427-34.
8. Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952;14(3):413-20.
9. Perrino C, Prasad SV, Mao L, Noma T, Yan Z, Kim HS, et al. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116(6):1547-60.
10. Carroll SM, Nimmo LE, Knoepfler PS, White FC, Bloor CM. Gene expression in a swine model of right ventricular hypertrophy: Intracellular adhesion molecule, vascular endothelial growth factor and plasminogen activators are upregulated during pressure overload. J Mol Cell Cardiol. 1995;27(7):1427-41.
11. Bishop SP, Melsen LR. Myocardial necrosis, fibrosis, and DNA synthesis in experimental cardiac hypertrophy induced by sudden pressure overload. Circ Res. 1976;39(2):238-45.
12. Siehl DL, Gordon EE, Kira Y, Chua BHL, Morgan HE. Protein degradation in the hypertrophic heart. In: Glaumann H, Ballard FJ, eds. Lysosomes: their role in protein breakdown. London:Academic;1987.
13. Zimmer HG, Ibel H, Gerlach E. Significance of the hexose monophosphate shunt in experimentally induced cardiac hypertrophy. Basic Res Cardiol. 1980;75(1):207-13.
14. Takahashi Y, Nakano S, Shimazaki Y, Kadoba K, Taniguchi K, Sano T, et al. Echocardiographic comparison of postoperative left ventricular contractile state between one and two-stage arterial switch operation for simple transposition of the great arteries. Circulation. 1991;84(5 Suppl.):III180-6.
15. White FC, Nakatani Y, Nimmo L, Bloor CM. Compensatory angiogenesis during progressive right ventricular hypertrophy. Am J Cardiovasc Pathol. 1992;4(1):51-68.
16. Kassab GS, Imoto K, White FC, Rider CA, Fung YC, Bloor CM. Coronary arterial tree remodeling in right ventricular hypertrophy. Am J Physiol. 1993;265(1 Pt 2):H366-75.
17. Le Bret E, Lupoglazoff JM, Borenstein N, Fromont G, Laborde F, Bachet J, et al. Cardiac "fitness" training: an experimental comparative study of three methods of pulmonary artery banding for ventricular training. Ann Thorac Surg. 2005;79(1):198-203.
18. Borenstein N, Jian Z, Fromont G, Bruneval P, Hekmati M, Behr L, et al. Noncultured cell transplantation in an ovine model of right ventricular preparation. J Thorac Cardiovasc Surg. 2005;129(5):1119-27.
19. Mavroudis C, Backer CL. Arterial switch after failed atrial baffle procedures for transposition of the great arteries. Ann Thorac Surg. 2000;69(3):851-7.
20. Boutin C, Wernovsky G, Sanders SP, Jonas RA, Castaneda AR, Colan SD. Rapid two-stage arterial switch operation. Evaluation of left ventricular systolic mechanics late after an acute pressure overload stimulus in infancy. Circulation. 1994;90(3):1294-303.








 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license