Objective: The prognosis of congenital complete heart block (CHB) is very poor when manifested with fetal hydrops. Fetal pacing may improve the survival rate. This study aims to assess the electrophysiological characteristics of a new lead, as well as fetal hemodynamic and metabolism under different fetal heart rates.
Methods: The new lead (T-shaped) was deployed into the myocardium of five fetal goats. Fetal cardiac output was measured by a flow transducer. CHB was achieved by cryosurgical ablation of fetal AV node. Electrophysiological parameters, hemodynamic and metabolic behavior of the fetus under different fetal heart rates were evaluated.
Results: The acute stimulation thresholds were consistently low. The voltage strength-duration curve remained relatively constant at pulse widths > 0.5 msec. The stimulation resistance was 1050.4 ± 76.6 ohms, and the sensed fetal R wave was 8.6 ± 5.6 mV. Fetal heart rate bellow 60 bpm was associated to low cardiac output and low blood pressure (p<0.05). Fetal bradycardia also determined oxygen saturation drop parallel to low cardiac output, with severe hypoxia below 60 bpm.
Conclusions: The new lead allows for a less invasive procedure and stable fixation to the myocardium that may prevent lead dislodgement after fetal recovery. It seems to be compatible with safe chronic stimulation. This study suggests that a gradual increase in the fetal rate beginning on 80 bpm after implantation would be more adaptive and adequately augment fetal cardiac output.
Objetivos: O bloqueio atrioventricular total (BAVT) fetal apresenta mau prognóstico quando associado à hidropisia. O implante de marca-passo pode melhorar as chances de sobrevida fetal. Este estudo analisa as características eletrofisiológicas do novo eletrodo de marca-passo fetal, a hemodinâmica e metabolismo fetal sob freqüências cardíacas variadas.
Método: O novo eletrodo (formato de "T") foi implantado no coração de cinco fetos de cabras. O débito cardíaco fetal foi medido com fluxômetro. O BAVT foi obtido por meio da crioablação do nó atrioventricular fetal. Os parâmetros eletrofisiológicos, hemodinâmica e metabolismo fetal foram analisados sob diversas freqüências cardíacas.
Resultados: O eletrodo fetal apresentou baixos limiares agudos, sem falhas no comando do estímulo. A curva do limiar de estimulação permaneceu relativamente constante para larguras de pulso acima de 0,5 mseg. A resistência foi de 1050,4 ± 76,6 ohms; e a onda R foi de 8,6 ± 5,6 mV. A freqüência cardíaca fetal abaixo de 60 bpm produziu baixo débito cardíaco fetal e hipotensão arterial (p<0,05). A bradicardia fetal ocasionou queda da saturação de oxigênio paralela à queda do débito cardíaco fetal, sendo mais grave abaixo de 60 bpm.
Conclusões: O novo eletrodo permite um procedimento menos invasivo e uma fixação miocárdica estável, evitando-se o deslocamento do eletrodo após a recuperação da atividade fetal. Os limiares obtidos são compatíveis com uma estimulação crônica estável e segura. Este estudo sugere a freqüência cardíaca inicial de 80 bpm pós-implante, com posterior aumento gradual, o que permitirá um aumento adequado do débito cardíaco e uma situação mais adaptativa.
INTRODUCTION
According to a multicentric study carried out in Europe and the United States, congenital complete heart block (CHB) is seen in one in every 20,000 live births, with 70% occurring in isolation and 30% associated to other congenital heart diseases [1]. Left atrial isomerism is a congenital malformation commonly associated with CHB (between 86% and 91% of cases) [2]. Taking into account the intra-uterine mortality rate, it is probable that the prenatal incidence of CHB is at least two times this, that is, approximately one in every 10,000 pregnancies.
Generally, CHB is well tolerated if there are no systemic or heart anomalies or placental insufficiency. However, prognosis is dismal when CHB is associated with fetal hydrops, evidenced by the accumulation of liquids in more than one cavity of the fetus, as a consequence of frequency-dependent heart insufficiency [3]. A multicentric study on the natural history of congenital CHB demonstrated that fetal hydrops occurs in 22 (40%) of every 55 fetuses with this disease [4]. This morbid association of diseases has a lethal effect in all cases, independently of the presence of associated congenital heart diseases.
The physiopathologic arguments for pacemaker implantation in fetuses are convincing. However, this procedure using a c-section so the pregnancy can continue, sometimes results in premature childbirth, an undesired outcome for a baby with immature lungs and heart insufficiency. On the other hand, puncturing the amniotic cavity presents a small risk for fetal loss estimated at approximately 0.5% [5]. Probably, the puncture of the fetal myocardium with adequate resources, guided by ultrasonography, for the implantation of a pacemaker lead could be performed with low risk of fetal loss, too.
An electrophysiological assessment after implantation is essential to guarantee pacemaker control during pregnancy and this should be carefully analysed in any experimental model. The sensitivity and pacing represent specific functions of a pacemaker generator and these should be separately tested.
With the aim of minimizing the maternal-fetal surgical trauma and to avoid the necessity of c-sections for pacemaker implantation with the possibility of postoperative premature childbirth, a prototype lead for percutaneous fetal pacemaker implantation was developed in the Heart Institute, HC-FMUSP, Sao Paulo, Brazil [6,7].
The purpose of this study is to experimentally evaluate the electrophysiological characteristics of this new lead in an experimental model of congenital CHB, as well as to assess the fetal hemodynamics and metabolism under different heart rates.
METHOD
This study was performed according to the norms of animal use in teaching and research of the Animal Research Inspection Commission. The protocol was approved by the Scientific Commission of the Heart Institute and by the Ethics Committee of the Hospital das Clínicas of FMUSP.
Five pregnant goats with gestational ages between 90 and 120 days (60% to 80% of the gestation term of 147 days) and with a mean weight of 46.9 kg (range 39 to 56 kg) were used in this study.
The goats received, as antibiotic prophylaxis, a dose 1,200,000 IU of penicillin benzathine and 3 x 1g doses of cephalotin and 40 mg gentamicin intramuscularly (IM) every 12 hours starting immediately before the surgery.
Anesthesia
The animals were maintained fasting for 24 hours. Anesthesia was induced with ketamine (10 mg/kg) IM. Subsequently, a venous line was established through a puncture of the jugular vein to infuse drugs and a crystalloid solution. Anesthetic induction was completed using pentobarbital (100mg EV) for orotracheal intubation. The goats were placed in the dorsal horizontal decubitus position. Controlled mechanic ventilation (ventilator Harvard 708, South Natik, MA, EUA) was established and maintained with inspired oxygen fractions at 100% and a flow volume of 15 mL/kg, to sustain arterial oxygen saturation higher than 95%.
Anesthesia was maintained with inhalation of 0.5 to l% halothane and extra doses of ketamine (1 mg/kg EV) and/or pentobarbital (100mg EV) according to necessity.
Monitoring consisted of electrocardiography and measurement of maternal arterial pressure, which was obtained by catheter in the femoral artery and continuously recorded using a computer (Biopac Systems, Inc., Goleta, CA, EUA) and interpretation software ACQknowledge 3.01.
Fetal anesthesia was achieved by an injection of ketamine (50 mg/kg IM), immediately after opening of uterine cavity, as well as from halothane, which easily passes through the placental barrier [8].
Procedure
The goat was prepared for a sterile surgical intervention. The womb was exposed by infra-umbilical median laparotomy. After hysterotomy of 10 cm above the right front limb of the fetus, the amniotic fluid was suctioned. Then the right axillary region of the front limb was exposed. The artery of the limb was cannulated using a 17-G intracath to monitor the systemic arterial pressure and to collect blood for analysis of the blood gases (New Biomedical - Stat Profile Ultra; Waltham, MA, EUA).
Thereafter, the fetal heart was exposed by medium transsternal thoracotomy. A basal ECG was obtained by means of three leads implanted into the wall of the fetal thorax. After opening the pericardial sack, the pulmonary trunk was dissected and isolated for the implantation of a T 106 ultrasonic flowmeter (Transonic Systems Inc., Ithaca, NY, EUA). Subsequently, the new lead was implanted as follows:
New lead
Figure 1 shows the new lead illustrating in detail its tip with a "T-shape" bar, to be inserted on the fetal myocardium. The lead consists of a multifilament bipolar parallel flexible twisted steel wire (242 cm), with the negative pole insulated with blue polyethylene and the positive with red. The end of the negative wire (blue) has a small "T-shape" metallic bar (4.0 x 0.4 mm) to assist insertion and fixation in the fetal myocardium. Thus, the new lead is firmly anchored in the fetal myocardium, preventing its displacement after recovery of fetal activity. The end of the positive wire (red), which is slightly shorter than the negative pole, terminates in a 4.0 mm metallic tip 5.0 mm away from the metallic "T-shape" bar of the negative pole.
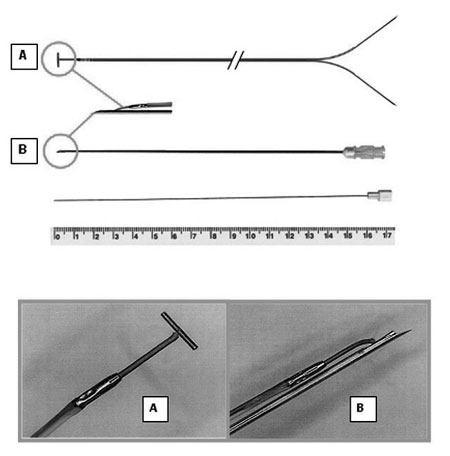
Fig. 1 - Prototype of the needle with the "T-shape" bar tip: (A) the 4.0 x 0.4 mm metallic bar; (B) 20-G needle modified at the distal end by a 25º bevel and extension of 5.0 mm. Below, mandrel to release the lead
The implantation of this lead is guided by the tip of a 14.84-cm 20-G needle with a 5-mm beveled distal end which only allows the introduction of the metallic "T-shaped" bar of the lead within the needle. The lead is then held parallel to the needle. After puncturing the fetal heart, the new lead is released from the needle using a mandrel introduced into the needle. So, the lead remains fixed in the myocardium, while the needle is removed with the mandrel. The other end of the lead has two long straight probes (positive and negative poles) to connect to the pulse generator.
The new lead was implanted on the right ventricle of the fetal after puncturing with a 20G needle and anchored to the fetal myocardium using a prolene 7-0 thread.
Fetal complete heart block
After implantation, the lead was connected to an ERA 300 pulse analyzer (Biotronik, Berlin, Germany) to measure the sensitivity threshold (R wave and slew rate) before inducing a block. Resistance was measured at a constant voltage (5 V) and a 0.5 msec pulse width. Subsequently, the pulse analyzer was programmed to stimulate the fetal heart at a frequency of 80 beats per minute during the CHB procedure.
With continuous electrocardiographic monitoring, fetal CHB was obtained by cryoablation of the atrioventricular node without establishing a cardiopulmonary bypass [9,l0]. A 3-mm cryoablator tip (Frigitronics Cryosurgical System CCS100, Shelton, CT) was applied on the wall of coronary sinus ostium, compressing it against the tricuspid valve annulus. The area was frozen to -40ºC for 60 seconds and after reheated. Fetal CHB was confirmed by a visual inspection for atrioventricular dissociation and the ECG was recorded continuously. When CHB was obtained, ventricular pacing was initiated at a frequency of 140 bpm.
Pacing threshold of the fetal myocardium
Measurements were made with a constant pulse width until the lowest voltage gave a 100% ventricular capture ratio. The pacing thresholds were measured with pulse widths from 0.1 msec to 2.0 msec, with a gradual reduction of the pulse analyzer voltage until asystole occurred. Then, the current for that specific threshold was measured. After all the measurements, the pulse analyzer was adjusted to the following parameters: mode VVI, sensitivity l.25 mV, pulse amplitude 5.0 V and refractory period 400 msec.
Hemodynamical and fetal metabolic assessment
Before inducing CHB, the blood flow of the fetal pulmonary trunk was measured. After CHB and assessing the pacing thresholds of the new lead, the heart rate was randomly varied from 40 to 140 bpm for hemodynamical and metabolic evaluation, with a minimal interval of 5 minutes for each frequency to check the variables. Subsequently the flow in the fetal pulmonary trunk was measured and fetal blood samples were drawn for analyses of gases and pH.
Fetal cardiac output was calculated from fetal pulmonary trunk blood flow, remembering that the right ventricle produces approximately 40% of the total fetal cardiac output. The fetal cardiac output was indexed to the fetal body weight.
After terminating the protocol, both the fetus and the heart were weighed. The womb and abdominal wall were closed with running sutures of absorbable thread. After complete recovery from the anesthesia and extubation, the goats were taken to the animal house on a special stretcher.
Statistic analysis
Comparison of measurements at specific points in time of fetal cardiac output caused by the different fetal heart rates was achieved using Friedman's non-parametric test complemented by Dunn's test for multiple comparisons. All values were expressed as means ± standard deviation. The level of significance utilized for the tests was 5%.
RESULTS
The fetuses had a mean weight of 1.78 kg ± 0.73 kg. CHB was attained in all fetuses, although three of them spontaneously reverted to sinus rhythm minutes after cryoablation of atrioventricular node. For these cases the procedure was repeated for 90 seconds when definitive complete blocks were achieved. In one of the fetuses a ventricular escape rhythm of more than 50 bpm was observed after induction of CHB, thereby impeding hemodynamical and metabolic assessment with a heart rate of less than 60 bpm.
Arterial gases and hematocrit levels in fetuses obtained before the procedure remained within physiological limits. There were no maternal hemodynamical alterations resulting from the procedure, with arterial pressure and blood gas levels stable during the protocol.
Electrophysiological analysis of the new lead
The implantation of the lead did not give technical difficulties. There were no occurrences of diaphragmatic or phrenic nerve stimulation.
In respect to sensitivity parameters, the mean measurement of the R wave was 8.64 ± 5.61 mV, while the slew rate was 1.64 ± 2.07 V/sec. Stimulation resistance was 1050.40 ± 76.60 W.
Acute pacing thresholds of the new lead were constantly low, without failure in pacing command. Figure 2 shows the tension curve by pulse width. The curve remained relatively constant with pulse width values of more than 0.5 msec.
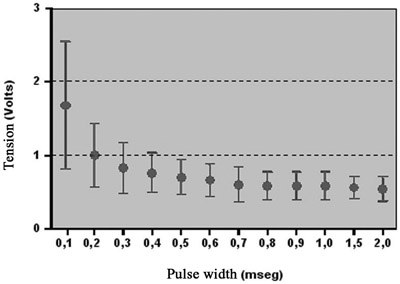
Fig. 2 - Tension curve x pulse width (mean ± SD) of the new lead for acute pacing threshold of the fetal myocardium. N = 5, values = mean ± standard deviation
There was an increase in acute pacing thresholds inversely proportional to pulse width values of less than 0.5 msec.
Hemodynamical alterations
Before induction of CHB, the initial fetal cardiac output was 444.84 ± 228.76 mL/min/kg for a heart rate of 149.20 ± 18.07 bpm. Figure 3 shows the relationship between the fetal cardiac output and the different heart rates. There were significant differences for the fetal cardiac output between specific points in time (p=0.0004). Comparing the points in time in pairs, significant differences were observed between the basal heart rate and heart rates of 40, 50 and 60 bpm (p< 0.05). Other times did not give significant differences.

Fig. 3 - Relationship between fetal cardiac output and fetal heart rate. N = 5, Values = mean ± standard deviation Friedman test: p = 0.0004
A drop in fetal cardiac output of up to 40% was observed with pacing frequencies of less than 60 bpm. On the other hand, frequencies of 120 and 140 bpm gave a drop in cardiac output of less than 20%. However, total recovery of the cardiac output compared to the basal level was not observed with the highest frequency (140 bpm).
The evolution of the systemic arterial pressure at different heart rates is demonstrated in Figure 4A. The mean fetal systolic pressure was 48.25 ± 2.63 mmHg, while the diastolic pressure was 39.75 ± 3.86 mmHg. Parallel to the cardiac output, the systemic arterial pressure of the fetus suffered a reduction with pacing frequencies of less than 60 bpm, while at the highest frequencies (120 and 140 bpm) there was a greater similarity to the basal arterial pressure.
The percentage variations of the fetal systolic and diastolic arterial pressures to different heart rates compared to the initial arterial pressure are demonstrated in Figure 4B. The drop in fetal diastolic pressure was greater than the fall in systolic pressure at heart rates of less than 60 bpm.
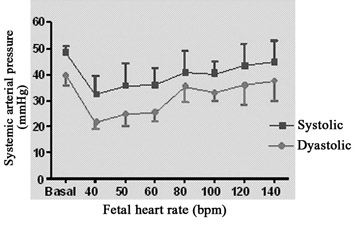
Fig. 4A - Relationship between fetal systemic arterial pressure and fetal heart rate. N = 4. Values = mean ± standard deviation
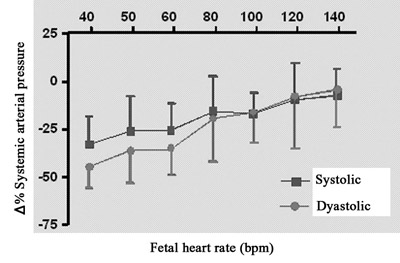
Fig. 4B - Relationship between percentage variation of fetal systemic pressure and fetal heart rate. N = 4. Values = mean ± standard deviation
The behavior of fetal blood oxygen saturation in comparison with fetal heart rate is shown in Figure 5A. There was a reduction in the oxygen saturation directly proportional to the reduction in fetal heart rate. Nevertheless, the higher heart rates did not give a total recovery of oxygen saturation in comparison to the initial level.
The relationship between the changes of fetal oxygen saturation compared to heart rate is demonstrated Figure 5B. There was a significant drop in oxygen saturation (up to 70%) with the lowest heart rate (40 bpm), whilst a drop of 31.35% in comparison to the basal oxygen saturation was observed at the highest fetal heart pacing frequency (140 bpm).
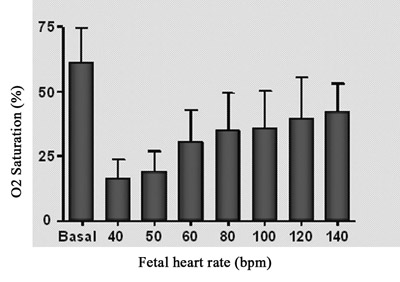
Fig. 5A - Relationship between fetal oxygen saturation and fetal heart rate. n=4, Values= mean ± standard deviation
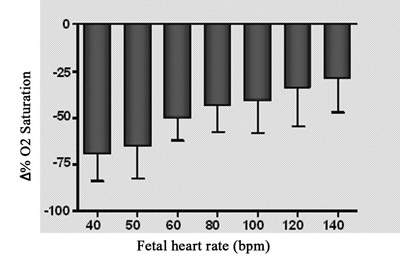
Fig. 5B - Relationship between percentage variation of fetal oxygen saturation and fetal heart frequency. N = 4. Values = mean ± standard deviation
Fetal blood pH ranged from 7.15 ± 0.10 (heart rate of 40 bpm) to 7.27 ± 0.14 (heart rate of 80 bpm), remaining in respiratory acidosis during all the protocol of artificial pacing (Table 1).
DISCUSSION
CHB remains a complicated disease, specifically when associated to very low heart rates (< 50 bpm) and fetal hydrops with high fetal and neonatal mortality rates [11]. When low output or heart rates cannot be reverted with clinical treatment, this severe heart condition nearly always results in intra-uterine death. Survivors are submitted to temporary pacemaker implantation during neonatal recovery. However, mortality with this type of therapeutic approach is still very high (approximately 80%) [12]. Due to the poor prognosis for congenital CHB associated with fetal hydrops, fetal pacemaker implantation can represent a great chance of survival. This therapeutic alternative offers some advantages: Firstly: definitive treatment can be introduced immediately after the first signs of fetal hydrops. This would allow the pregnancy to continue normally with recovery from heart insufficiency and normal fetal development until full term, with stable respiratory and cardiovascular functions during delivery.
When there is echocardiographic evidence of deterioration of the hemodynamic parameters, development of or increase in hydrops, in spite of clinical treatment, the next logical step should be prenatal pacemaker implantation. Until recently, our capacity to surgically treat CHB in fetuses was very limited.
Currently, the new "T-shape" lead may be an interesting alternative for fetus percutaneous pacemaker implantation, without the necessity of performing fetal thoracotomy or hysterotomy. This system allows less invasive implantation and stable myocardial anchoring, avoiding lead displacement after recovery of fetal activity. However, before its use, it is important to evaluate the lead under electrophysiological conditions in an experimental model of CHB to assess its clinical applicability. The hemodynamical and metabolic assessments of the ideal initial frequency of ventricular pacing during implantation are also necessary for the best adaptation of the fetus to the new conditions of artificial pacing.
In the mid 1990s, an experimental model of CHB in sheep fetuses was developed in our laboratory [10,11], obtained by cryoablation of the atrioventricular node, without using cardiopulmonary bypass. It is a simple and reproducible method of assessing fetal pacemaker leads which is already being used by other research centers in Japan [13, 14] and the United States [15] for studies on the physiopathology of artificial fetal heart pacing. Our studies demonstrated that cryolesions of the atrioventricular conductor system do not cause septal myocardial infarction [16]. Maintenance of the hemodynamical balance after CHB can be related to the absence of septal myocardial infarction and the focal pattern of the cryoblation lesion on the atrioventricular node.
The sensitivity parameters (R wave and slew rate) confirmed the satisfactory performance of the new lead. The low fetal pacing thresholds and resistance demonstrated in the current study are similar to the values expected from a conventional pediatric system. They are totally compatible with safe chronic pacing up to birth with the possibility of good pulmonary development. Capture was stably achieved in all experiments. A consistent chronic capture is desirable for all child and adult pacing systems. Fetal systems are normally used for a few weeks or some months of pregnancy, sufficient time for fetal lung development and to solve the complications of hydrops. Dell'Orfano et al. [17] suggest that artificial fetal myocardial pacing must be used for at least two to four weeks before c-section to effectively reduce the anasarca and pulmonary edema. After birth the system is substituted for a conventional epicardial system.
Another interesting characteristic of the new fetal pacemaker system is the bipolar stimulation directly in the fetal myocardium, thus avoiding electric current from the lead stimulating uterine musculature. Pacemaker stimulation of the myometrium may contribute to early labor. We chose ventricular stimulation due to the simplicity of lead implantation into the small fetal heart and because of the risks inherent with the puncture of the fine atrial wall.
An important limitation of the study is the fact that the animals had normal hearts and stimulation thresholds, differently to the human hearts that will be treated, although edema of tissues causes a lower resistance to pacing.
The use of the CHB model to measure fetal cardiac output at different heart rates provided an opportunity to examine immediate hemodynamical responses. This study demonstrates a drop in cardiac output directly proportional to the reduction in heart rate. This drop (significant at rates of 60 bpm and less) is associated to a drop in arterial pressure and oxygen saturation. Even at the highest heart rates with artificial ventricular pacing (140 bpm), we observed a drop of 16.06% ± 7.84%, in agreement with the findings of Kikuchi et al. [18]. Our findings are also corroborated by the studies of Liddicoat et al. [15], which demonstrated a drop of 12% in cardiac output associated to ventricular stimulation with atrial pacing of sheep submitted to intra-uterine pacemaker implantation. Probably, ventricular diastolic filling is prejudiced by atrioventricular asynchrony and by the loss of part of the atrial output at ventricular diastole, a more important situation in fetuses than in neonates [19].
On the other hand, although the Frank-Starling mechanism is present in fetuses [20, 21], alterations of the heart rate determine greater changes in the fetal ejection fraction when comparing the alterations of the fetal pre- and post-loads. The small fetal cardiac output associated to the frequency at less than 60 bpm reflects the limited capacity of the fetal heart to increase the ejection fraction during extreme bradycardia. Although the primary heart development is completed in about eight weeks of pregnancy, many cellular and molecular components remain immature until after birth. The fetal myocardial contractile elements are qualitatively and quantitatively different in the adult myocardium. The fetal myocytes present smaller diameters and the total number of sarcomeres per gram of fetal myocardium is considerably less than in the adult. Consequently, the fetal myocardium develops greater tension when expanded during diastole and thus is less complacent. Hence, the restricted response of ejection fraction of the fetal heart under extreme bradycardia may be related to immaturity of the fetal myocardium.
In adult animals, on the other hand, the ejection fraction is adjusted to great alterations of heart rate, maintaining the cardiac output relatively constant. Thus, the fetal ventricle is very dependent on heart rate to maintain appropriated cardiac output [22].
We must mention another limitation of the present study. The minimum heart rate to maintain a reasonable cardiac output was demonstrated in an acute experimental model, which surely can not be compared to the condition of a human fetus that gradually evolves with CHB. Furthermore, the exteriorization of the thorax to perform the thoracotomy, the dissection of great vessels and the cryoblation of atrioventricular node, probably influenced the cardiovascular function.
Although the number of preparations is small, it is possible that there is a tendency to proportionally reduce the diastolic pressure greater than the systolic pressure at heart rates of less than 60 bpm. This fact may favor inadequate coronary perfusion during extreme fetal bradycardia, with important implications for the higher fetal mortality rates due to CHB at heart rates under 55 bpm, as was observed in a multicentric study by Schmidt et al. [4].
Invariably changes in fetal heart rates determined similar alterations in fetal oxygen saturation parallel to a drop in cardiac output. These observations have important clinical implications. It was possible to evaluate the magnitude of the drop in fetal cardiac output from the reduction of fetal heart rate and its consequences on placental gas exchange. The drop in fetal cardiac output had as a consequence a reduction on the placental blood flow, causing harmful changes in hematosis and consequently respiratory acidosis. The drop in oxygen saturation was more severe in pacing frequencies of less than 60 bpm.
We speculated that deterioration of the preparation plays an important role on respiratory acidosis, as the assessment of blood gases was made after a long exposure, due to technical difficulties to prepare for the study protocol. It is possible that fetal manipulation and surgical stress during preparation had a cardiodepressant action, as persistent acidosis and a drop of oxygen saturation was noted at all heart rates. The reduction in systemic blood flow aggravates even more respiratory acidosis due to inadequate distribution of oxygen to the tissue. Thus, observations of the effects of the changes in heart rate with fetal CHB give a notion of fetal cardiocirculatory adaptability and its repercussions on the fetal metabolism.
Clinical implications
The possibility of continuing the pregnancy and preventing premature labor with its consequences by using the new lead can contribute to better results in the treatment of congenital CHB conferring significant hemodynamic repercussions.
The lead for percutaneous pacemaker implantation in fetuses described in this work can offer significant technical advantages in comparison with leads used in conventional open-surgery procedures. The new "T-shape" lead provides stable anchoring with satisfactory performance, demonstrated by the small pacing thresholds. Moreover, this technique potentially minimizes surgical trauma to the mother and fetus. It is also probably less expensive, simpler and faster than conventional epicardial lead implantation.
Harrison's group, from the University of California, São Francisco [23], and Zielinsky (personal communication), from the Heart Institute of Porto Alegre, Brazil, tried open-surgery pacemaker implantation in human fetuses using c-sections but both resulting in lethal intra-operative outcomes.
Probably, puncture of the fetal myocardial for pacemaker lead implantation, with suitable resources and guided by ultrasound can be performed with low risk of fetal loss.
Previous attempts of intra-uterine pacemaker implantation by puncture, either via a transthoracic approach [24] or by the inferior vena cava [25] resulted in fetal death a few hours after the procedure. Although the authors have demonstrated that leads can be suitably positioned within the heart, the lack of myocardial anchoring and consequent lead displacement after recovery of fetal activity represents the greatest technical limitation of any percutaneous approach. The authors have demonstrated that leads can be suitably positioned within the fetal heart, they did not manage to prevent displacement after recovery of fetal activity.
In the reported case of pacemaker implantation in a human fetus [6,7], the following question appeared only after placement of the lead in the fetal myocardium: What should be the initial pacing frequency of a fetal heart? It was decided to suddenly increase the rate from 47 bpm to 140 bpm. According to Schmidt et al. [4], a heart rate of over 55 bpm is well tolerated by a fetus. In the present study, heart rates of less than 60 bpm gave a significant drop in heart output (> 40%), while heart rates greater than 80 bpm produced a lower reduction in fetal cardiac output (< 26%). It seems that a gradual increase in heart rate, after pacemaker implantation in the fetus would be more sensible and could suitably increase the fetal cardiac output. Perhaps a sudden increase in fetal heart rate can result in metabolic imbalance, due to the abrupt increase in tissue and myocardial oxygen consumption, impairing the postoperative evolution. Although the studies of Ayustawati et al. [13] demonstrated, using our experimental model of CHB, a better hemodynamical performance with a pacing frequency of 150 bpm, the current study suggests that the fetal pacing frequency, at the time of implantation, should be initiated at 80 bpm and gradually increased during the pregnancy to allow a more adaptable situation.
CONCLUSIONS
The new lead of fetal pacemaker allows a less invasive procedure and a stable myocardial anchoring, avoiding displacement of the lead after recovery of fetal activity. It presented a satisfactory electrophysiological performance, with low acute thresholds, compatible with a stable and safe long-term pacing. This study also demonstrated that the alterations in fetal heart rate, in an experimental model of CHB with artificial direct ventricular pacing, produced low fetal cardiac output and arterial hypotension, specifically at frequencies of less than 60 bpm. The reduction of fetal heart rate caused a drop in fetal blood oxygen saturation parallel to a drop of fetal cardiac output which was more severe at pacing frequencies of less than 60 bpm. This study suggests an initial heart rate of 80 bpm post-implantation, with a gradual subsequent increase allowing a suitable increase in the cardiac output and a more adaptive situation.
ACKNOWLEDGMENTS
We would like to thank Dr. Sérgio Galbinsky for his valuable collaboration during the protocol and Richard Barbosa da Silva, laboratory technician and Michael Lee Biotronik engineer for their immeasurable support during data collection for this study.
REFERENCES
1. Waltuk J, Buyon JP. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544-51.
2. Lopes LM, Cha SC, Sadek L, Iwahashi ER, Aiello VD, Zugaib M. Bloqueio atrioventricular fetal. Arq Bras Cardiol. 1992;59(4): 261-4.
3. Eronen M, Heikkila P, Teramo K. Congenital complete heart block in the fetus: hemodynamic features, antenatal treatment, and outcome in six cases. Pediatr Cardiol. 2001;22(5):385-92.
4. Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol. 1991;17(6):1360-6.
5. Golbus MS, Loughman WD, Epstein CJ, Halbasch G, Stephens JD, Hall BD. Prenatal genetic diagnosis in 3000 amniocenteses. N Engl J Med. 1979;300(4):157-63.
6. Assad RS, Zielinsky P, Kalil R, Lima G, Aramayo A, Santos A et al. Novo eletrodo para implante de marca-passo em fetos com bloqueio atrioventricular total. Rev Bras Cir Cardiovasc. 2003;18(1):40-4.
7. Assad RS, Zielinsky P, Kalil R, Lima G, Aramayo A, Santos A et al. New lead for in utero pacing for fetal congenital heart block. J Thorac Cardiovasc Surg. 2003;126(1):300-2.
8. Sabik JF, Assad RS, Hanley FL. Halothane as an anesthetic for fetal surgery. J Pediatr Surg. 1993;28(4):542-6.
9. Assad RS, Jatene MB, Moreira LFP, Sales PC, Aiello VD, Costa R et al. Bloqueio A-V total congênito: novo modelo experimental para avaliação do marca-passo fetal. Rev Bras Cir Cardiovasc. 1994;9(3):133-40.
10. Assad RS, Jatene MB, Moreira LF, Sales PC, Costa R, Hanley FL et al. Fetal heart block: a new experimental model to assess fetal pacing. Pacing Clin Eletrophysiol. 1994;17(7):1256-63.
11. Donofrio MT, Gullquist SD, Mehta ID, Moskowitz WB. Congenital complete heart block: fetal management protocol, review of the literature, and report of the smallest successful pacemaker implantation. J Perinatol. 2004; 24(2):112-7.
12. Kleinman CS, Donnerstein RL. Ultrasonic assessment of cardiac function in the intact human fetus. J Am Coll Cardiol. 1985;5(I suppl):84S-94S.
13. Ayustawati, Shiraishi H, Kikuchi Y, Hoshina M, Momoi MY, Sato I. Optimal ventricular pacing rate in fetal lambs with complete atrioventricular block. Am J Obstet Gynecol. 2002;186(5):1052-5.
14. Shiraishi H, Kikuchi Y, Hoshina M, Ohki T, Ayustawati, Momoi MY. Hemodynamic effect of the ventricular pacing site in fetal lambs with complete atrioventricular block. Pacing Clin Electrophysiol. 2002;25(12):1731-6.
15. Liddicoat JR, Klein JR, Reddy VM, Klautz RJ, Teitel DF, Hanley FL. Hemodynamic effects of chronic prenatal ventricular pacing for the treatment of complete atrioventricular block. Circulation. 1997;96(3):1025-30.
16. Assad RS, Aiello VD, Jatene MB, Costa R, Hanley FL, Jatene AD. Cryosurgical ablation of fetal atrioventricular node: new model to treat fetal malignant tachyarrhythmias. Ann Thorac Surg. 1995;60(6 suppl):S629-32.
17. Dell' Orfano J, Chou HA, Park D, Mirza H, Stys T, Mahan V et al. The monolithic fetal pacemaker: prototype lead design for closed thorax deployment. Pacing Clin Electrophysiol. 2003;26(4 pt 1):805-11.
18. Kikuchi Y, Shiraishi H, Igarashi H, Chunfeng L, Yanagisawa M. Cardiac pacing in fetal lambs: intrauterine transvenous cardiac pacing for fetal complete heart block. Pacing Clin Electrophysiol. 1995;18(3 pt 1):417-23.
19. Crombleholme TM, Longaker MT, Langer JC, Bradley SM, Duncan BW, Adzick S et al. Complete heart block and AV-sequential pacing in fetal lambs: the atrial contribution to combined ventricular output in the fetus. Surg Forum. 1989;40:268-71.
20. Anderson PA, Killam AP, Mainwaring RD, Oakeley AE. In utero right ventricular output in the fetal lamb: the effect of heart rate. J Physiol. 1987;387:297-316.
21. Pitlick PT, Kirkpatrick SE, Friedman WF. Distribution of fetal cardiac output: importance of pacemaker location. Am J Physiol. 1976; 231(1): 204-8.
22. Anderson PA, Glick KL, Killam AP, Mainwaring RD. The effect of heart rate on in utero left ventricular output in the fetal sheep. J Physiol. 1986;372:557-73.
23. Silverman NH, Kohl T, Harrison MR, Hanley FL. Experimental fetal surgery in the animal model and in the human fetus. In: Imai Y, Momma K, eds. Proceedings of the Second World Congress of Pediatric Cardiology and Cardiac Surgery. Armonk:Futura Publishing;1998. p.622-3.
24. Carpenter RJ Jr, Strasburger JF, Garson A Jr, Smith RT, Deter RL, Engelhardt HT Jr. Fetal ventricular pacing for hydrops secondary to complete atrioventricular block. J Am Coll Cardiol. 1986;8(6):1434-6.
25. Walkinshaw SA, Welch CR, McCormack J, Walsh K. In utero pacing for fetal congenital heart block. Fetal Diagn Ther. 1994;9(3):183-5.
COMMENTS
Assessment of a new lead for pacemaker implantation in fetuses with congenital complete heart block (CHB)
Domingo Braile (São José do Rio Preto, Brazil, domingo@braile.com.br). In first place, I would like to congratulate the authors for doing this experimental work, which allows an exponential increase in our knowledge. I would also like to stress the importance of the issue; the development of a simple and innovative short-term lead for the treatment of congenital atrioventricular block. However, I have some questions that, certainly, will improve our understanding of this new development.
1 - In 2003, the authors published in number 18(1) p. 40 to 44, of BJCVS, an article entitled "New lead for pacemaker implantation in fetuses with complete atrioventricular block", in which they implanted in a human fetus a lead similar to the one described here, by puncturing the maternal abdomen, passing through the wall of the womb and finally, reached the fetus heart, guided by ultrasound. The thresholds obtained were good and temporary pacing was established. During the evolution, heart tamponade occurred and the fetus died after 36 hours. The leads were well positioned in the heart and in the thorax. My question: There is already clinical experience showing the good performance of the lead, why did you decide to do experiments in pregnant goats using surgery, when it seems that the greatest challenge is implantation of the lead using a percutaneous approach?
Dr. Renato Assad: In the first place, we thank you for the pleasant comments about our research line, established at the start of 90s. The current study is about an improvement of the lead that has been previously utilized in human fetuses (unipolar), as reported in an earlier publication. The most recent lead consists of parallel wires (bipolar) with the same idea as the preceding lead, that is, the tip terminates in a 'T-shape'. As it is bipolar, the electrical flow is limited to pacing of the fetal organism, thus avoiding myometrium stimulation and uterine contractions, with consequent premature parturition. Electrophysiological assessment during the implantation is fundamental to assure the pacemaker commands during pregnancy and this must be carefully analysed in the experimental model. The necessity of clinical application of the lead appeared soon after the conception of the prototype; an earlier experimental assessment was not possible. As the current prototype is thinner (introducer: 20G needle) than the previous model (17G needle), the chances of tamponade may be minimized.
Dr. Braile: 1) Is this a preliminary study to be continued with transuterine introduction?
Dr. Assad: No doubt, the next step of this research line will be an assessment of the chronic thresholds, with the lead percutaneously implanted in goat fetuses.
Dr. Braile: 2) Considering the premise,
a) Where is the ideal location of the thoracic puncture of the fetus? b) Is the left ventricle the ideal chamber to pace?
Dr. Assad: Leads will be placed in right and left ventricles, as they both have acceptable command thresholds for the fetal heart rhythm, compatible with safe chronic pacing.
Dr. Braile: b) Is there any way of avoiding puncturing the coronary artery, which would be lethal?
Dr. Assad: Although rare, it will not be possible to avoid puncturing the coronary artery, as the procedure will not be performed under open surgery to access the surface of the fetal heart.
Dr. Braile: c) What we understand from the description in the previously published article, the T-shape end of the lead is metallic. Would it be possible for this to be of a plastic material, which may reduce the area of the lead and make its removal much easier as it would be more flexible?
Dr. Assad: The problem is related to the movement of the fetus after recovery, when displacement of the lead may occur. The lead must remain firmly fixed in the myocardium.
Dr. Braile: d) Similar to the authors, I was also concerned about the possibility of pulling, twisting and displacement of the lead floating in the amniotic liquid; from the discussion in the article, I see that you already thought about obtaining a longer lead. Will you be able to demonstrate its use?
Dr. Assad: Maybe, we will be able to check the safety in respect to displacement of the lead only with clinical experience.
Dr. Braile: e) I understood that this lead will be temporary. What would the procedure be during a c-section and soon after delivery? Would another lead be implanted with another pacemaker? In this case, would the pacemaker be outside or already implanted in the newborn baby?
Dr. Assad: During c-section, the pulse generator previously implanted in subcutaneous tissue of the maternal abdomen would be extracted and the command of heart rhythm would be transferred to an external stimulator. At the moment of delivery, the wires would be briefly disconnected from the stimulus generator, to follow the fetus through the surgical incision (c-section). Afterwards, the leads would be reconnected to an external pulse generator for elective planning of definitive pacemaker implantation.







 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license