INTRODUCTION
Heart myxoma is a benign tumor; the most common primary tumor of the heart responsible for around 50% of all cases [1]. In general, 75 to 80% of myxomas are located in the left atria, 18% in the right atrium and much less frequently in the ventricles or centrally [2]. Patients present with at least one characteristic of a classically described triad that include: heart obstruction, constitutional symptoms and embolic events [3]. They can also present with arrhythmia due to the direct infiltration of the heart conduction tissue irritating the myocardium itself. Right atrial myxoma, in particular, can obstruct the cuspid valve, causing symptoms of right heart insufficiency, peripheral edema, hepatic congestion and syncopes [3].
Our objective is to report the case of a right atrium myxoma with prolapse to the right ventricle (RV) through the tricuspid valve.
CASE REPORT
A 67-year-old Caucasian was hospitalized with complaints of fatigue and adynamia over a three-month period. She reported over this time, mild dyspnea which improved with rest, a non-productive cough without hemoptysis and two episodes of cervical plethora, which improved spontaneously. She also reported two episodes of mild fever of 38 ºC without defined etiology forty-five days previously which the patient attributed to influenza. She also reported a history of asthma, systemic hypertension treated with 20 mg/day enalapril, total mastectomy of the right breast performed 5 years previously associated with chemotherapy and radiotherapy to treat cancer. A heartbeat of 100 beats per minute and blood pressure of 130 by 80 mmHg were recorded during the physical examination. The patient was afebrile and without central or peripheral cyanosis. She presented with physiological cardiopulmonary auscultation and there were no signs of masses or visceromegaly on abdominal palpation.
The hematological laboratory tests did not show any abnormalities except for increases in the hemosedimentation velocity (HSV) and the C-reactive protein, at levels of 114 mm and 6.47 mg/dL, respectively. All the other routine laboratory examinations were within normal ranges. A preoperative electrocardiograph did not demonstrate any alterations. A chest radiogram showed an increase in the cardiovascular silhouette and masking of the left diaphragmatic dome.
A computed tomography (CT) was performed of the chest with venous contrast that demonstrated a small pleural effusion on the left, a 2-mm thick pericardial effusion, right mastectomy and an apparent failure to fill the right atrium. A CT of the cranium was also performed that did not show any changes and a CT of the abdomen and pelvis showing a right renal cortical cyst (Bosniak I), small splenic nodule and aortic atheromatosis. A transthoracic echocardiogram demonstrated a mass measuring approximately 2.5 x 5.5 cm inside the right atrium which moved through the tricuspid valve to the right ventricle during diastole and a paradoxical movement of the atrial septum compatible with myxoma of the right atrium (Figure 1). A coronarography to assess possible coronary artery disease did not show abnormalities.
With the diagnostic hypothesis above, the patient was referred to the Heart Surgery Service and submitted to exeresis of the tumor utilizing the following technique: 1. longitudinal transsternal mediastinotomy; 2. longitudinal pericardiotomy taking care with the accentuated increase in the right atrium and the other normal heart structures; 3. establishment of a cardiopulmonary bypass (CPB) utilizing a Braile Biomedica Membrane Oxygenator; 4. Anticoagulation was achieved using 400 IU/kg of weight of sodium heparin; 5. The CPB was established by means of an arterial cannula placed at the end of the ascending aorta. Drainage of the inferior vena cava was achieved by the placing of a venous cannula in the right atrium, one centimeter above its origin. Drainage of the superior vena cava was achieved by placing the cannula inside the vein one centimeter below its root in the right atrium; 6. After aortic clamping, anterograde myocardial protection with isothermal and hyperkalemic oxygenated blood was initiated; 7. following cardiac arrest, with blood deviated completely from the right atrium, right atriotomy was performed exposing the voluminous tumoral mass without adherences to the heart structures (Figure 2), however with its pedicled base inserted in the interatrial septum near to the remnant of the oval foramen; 8. Exeresis was achieved by removing all the septal area connected to the tumor with subsequent septoplasty utilizing a bovine pericardial patch; 9. After closing the right atrium, the air was removed from the heart chambers in the normal way and the pulmonary and coronary circulation was restored; the heart returned to sinus rhythm; 10. With the normalization of the body temperature, the CPB was disconnected and after the cannulae were removed from the vena cavae, the heparin was neutralized by the administration of protamine chloride at 1 mg for each 100 IU of heparin utilized for anticoagulation. After the surgery was completed, the tumor was measured (2.5 x 5.5 cm) and sent for histopathologic which confirmed the diagnosis of myxoma. The immediate postoperative course was event free with the patient staying in the ICU for the first two postoperative days and being released from hospital on the 7th postoperative day without signs or symptoms of heart failure.

Fig. 1 - Transthoracic echocardiogram showing a mass of approximately 2.5 x 5.5 cm inside the right atrium
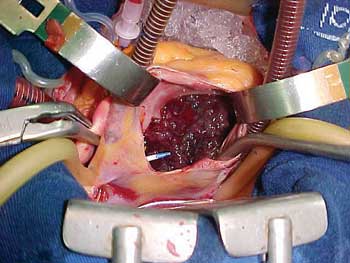
Fig. 2 - Right atrium myxoma - view of the voluminous tumoral mass after right atriotomy
Primary tumors of the heart are rare identified in 0.0017% of a series of autopsies [4]. Of these heart myxomas are the most common; it is a disease that affects patients within a broad age range (15-80 years), with a mean age of approximately 50 years old. There seems to be a slight predominance in women giving a ratio between women and men of 5:4 [3]. In spite of appearing to be benign, these tumors can course to a unfavorable evolution and are responsible for severe complications [1].
Due to the non-specific clinical presentation of patients with myxoma, the pre-mortem diagnosis was only identified in 1952 when Goldberg demonstrated a myxoma of the left atrium by means of cardiac angiography [3]. The first successful surgical resection of a cardiac myxoma was performed using CPB in 1954 and this patient continued to live until 1997 [3].
The most common symptom associated with heart myxoma is congestive heart failure, followed by either systemic or pulmonary embolization [5]. These symptoms, are generally related to the location of the tumor and vary in their intensity and form, as well as the physical activity and the position of the patient. In respect to myxoma of the right atrium, the clinical presentation can include ascitis, hepatomegaly or peripheral edema, all due to right heart insufficiency. Vague constitutional symptoms can also be found, such as feeling bad, fever (generally mild or long lasting) and weight loss [5]. These symptoms, as well as anemia, an increase in the HSV and the gammaglobulin serum levels may confuse the diagnosis [6] as they are due to an inflammatory response that can be associated to many diseases. In this case of right atrium myxoma, the patient presented with dyspnea on effort, a non-productive cough, episodes of cervical congestion and mild fever (38 ºC).
Although transthoracic echocardiography is less invasive and presents an excellent sensitivity of 95% for the detection of myxomas, the diagnostic sensitivity can be as high as 100% when succeeded by transesophageal echocardiography. CT and magnetic resonance may be useful to demonstrate the fixation point and associated complications. The recent progress in diagnostic methods, including the aforementioned examinations, has enabled the diagnosis of primary cardiac tumors without the use of heart catheterization or angiology, reliably identifying the real location of the tumor. Thus, cannulation of the vena cavae to establish CPB can be safely achieved through the right atrium, observing technical precautions in order to avoid dislodging the tumoral mass with consequent embolization specifically in cases in which there is a defect of the interatrial septum. Thus it is important to avoid cannulation of the femoral vein, thereby minimizing surgical complications.
The frequency of recurrence of cardiac myxomas varies from 3% in sporadic cases as reported here to 22% in cases associated with the "Carney complex" [4].
REFERENCES
1. Mota AAR, Colen Filho E, Colen EA, Vieira JAS, Alves MAP, Borges MF et al. Mixoma do átrio esquerdo: relato de 3 casos. Rev Bras Cir Cardiovasc. 1997;12(4):377-83.
2. Ipek G, Erentug V, Bozbuga N, Polat A, Guler M, Kirali K et al. Surgical management of cardiac myxoma. J Card Surg. 2005;20(3):300-4.
3. Grebenc ML, Rosado-de-Christenson ML, Green CE, Burke AP, Galvin JR. Cardiac myxoma: imaging features in 83 patients. Radiographics. 2002;22(3):673-89.
4. Acebo E, Val-Bernal JF, Gomez-Román JJ, Revuelta JM. Clinicopathologic study and DNA analysis of 37 cardiac myxomas: a 28-year experience. Chest. 2003;123(5):1379-85.
5. Murayama H, Tamaki S, Kato N, Yuji N, Yokote J, Mutsuga M et al. Right atrial myxoma associated with atrial septal defect: a case report and review of the literature. Ann Thorac Cardiovasc Surg. 2001;7(3):166-9.
6. Karachalios G, Bablekos G, Karachaliou I, Zoganas L, Charalabopoulos A, Charalabopoulos K. Left atrial myxoma prolapsing into the left ventricle: case report and review of the literature. Chemotherapy. 2004;50(6):297-301.


 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license