INTRODUCTION
Despite of great developments over recent years, bleeding continues to be one of the main morbidities in heart surgery, particularly with the advent of complex procedures with prolonged cardiopulmonary bypass and intervention times in acutely decompensated patients. As well as the higher hospital costs, blood and blood component transfusions and surgical re-interventions for hemostasis complications incurred, there are significant increases in the morbidity and mortality [1,2]. Thus, knowledge of risk factors for bleeding [3] is primordial to adopt preventive and therapeutic measures.
Due to the multifactorial nature of the problem, establishing universal protocols is necessary [4], but this is invariably more difficult due to the alterations in coagulation caused by cardiopulmonary bypasses (CPB). Several methods have been proposed in an attempt to minimize the blood loss in heart surgery, including the use of cell savers [5], autotransfusions [6], heparin-lined circuits [7], leukocyte filters [8] and antifibrinolytic drugs [9]. The inconvenience of these methods is in the high cost required to routinely implement them.
The objective of this study is to determine the risk factors for bleeding in the postoperative period of heart surgery, in order to identify high risk populations for future preventive measures.
METHOD
From October 2001 to March 2002, 411 consecutive patients submitted to the surgical treatment of heart disease were prospectively studied. Patients who were submitted to heart transplantation and those who passed away within the first 24 hours after the operation without mediastinal bleeding complications were excluded.
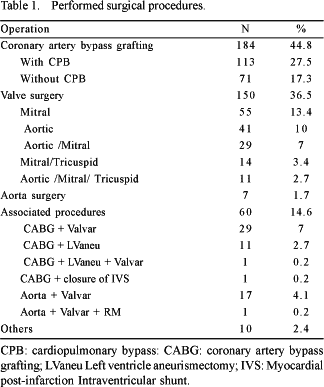
The mean age was 57.7 ± 13.9 years (varying from 14 to 91 years) and 59.6% of the patients were men. The mean weight was 69.l ± 14.1 kg (ranging from 32 to 130 kg). The surgical procedures (Table 1) included coronary artery bypass grafting in 227 (55.2%) patients, valve surgery in 198 (48.2%) and surgical treatment for aortic diseases in 25 (6.1%).
Sixty (14.6%) patients underwent combined surgical procedures with the majority being coronary artery bypass grafting together with valve surgery. Ten patients (2.4%) were submitted to several procedures, which included pericardiectomy (five patients), pulmonary thromboendarterectomy (three patients) and resection of a myxoma in the left atrium (two patients).
The most common approach was by median sternotomy, performed in 403 (98%) patients. Right antero-lateral thoracotomies were performed in eight young female patients for surgical treatment of mitral valve disease.
Cardiopulmonary bypasses were used in 335 (81.5%) patients. Before cannulization, heparin was administrated at 4 mg/kg of body weight in order to obtain an activated coagulation time (ACT) of greater than 480 seconds. The arterial blood flow was maintained at about 2.4 L/m2/min, in order to maintain the arterial blood pressure between 50 and 70 mmHg. Myocardial protection was achieved using sanguineous or crystalloid anterograde cardioplegia or intermittent aortic clamping, depending on the surgical team.
Ten (2.4%) patients were submitted to total circulation arrest (TCA) under deep hypothermia to treat type A aortic dissections, aortic arch aneurisms or chronic pulmonary thromboembolism. The mean TCA time was 35.3 ± 20.2 minutes (ranging from 10 to 70 minutes). After CPB interruption and priming volume replacement, the circulating heparin was neutralized by protamine administration at a proportion of 1:1.
Seventy-six (18.5%) patients underwent procedures without the use of CPB, including coronary artery bypass grafting (71 patients) and pericardiectomy (five patients). When heparin was necessary doses of from 2 to 3 mg/kg of body weight were employed in order to maintain the ACT two times the basal value. After the last anastomosis, the circulating heparin was neutralized by protamine administration at a proportion of 0.5:1.
Antifibrinolytic drugs were used for 148 (36%) patients, with aminocaproic epsilon acid used for 145 patients and aprotinin for three. Recommendation of use of these drugs included all patients submitted to reoperations and those submitted to their first operations but who were considered to be high risk for bleeding due to their preoperative characteristics. The type of antifibrinolytic utilized depended of the individual preferences of the anesthetist and surgical team. Epsilon aminocaproic acid was the drug of choice due to its low cost and results comparable to other antifibrinolytic agents [10,11]. The intraoperative autotransfusion using a cell saver was employed in 48 (11.7%) patients, more commonly with coronary artery bypass grafting surgeries without the use of CPB.
The volume of bleeding in the postoperative period was determined through the sum of losses through all thoracic drains from the moment of closure of the thorax over a period of 24 hours. Postoperative management was conduced by a multidisciplinary team, which included a cardiologist, an intensivist, a cardiovascular surgeon and a hematologist. The hematologist was responsible for blood component transfusion. There was no influence whatsoever of the authors of this study in respect to the correction of postoperative coagulation disorders or the indication of transfusions of blood or blood components or the surgical intervention for hemostasis. Each case was individually analysed in respect to the necessity of blood transfusions considering the hemodynamic conditions, the amount of bleeding, the patient's age and the history of hemorrhagic disorders. The professionals involved in the postoperative period did not have knowledge in respect to this study or its objectives.
In general, the following norms were adopted in respect to blood component transfusion. Significant bleeding (150 mL/hour) in the immediate postoperative period was treated according to the result of the coagulogram. Laboratory alterations, when bleeding was absent, did not indicate any type of treatment. The use of protamine was indicated when the partial activated thromboplastin (PAT) levels or activated coagulation time (ACT) when available, increased or when recirculation of heparin was suspected. When alterations of the prothrombin activity (PA) or in the international normalization ratio (INR) occurred, fresh frozen plasma transfusions were indicated. A platelet count below 100,000 per microliter with bleeding was indicative of transfusions of this component. A hematocrit level under 28% was corrected by concentrated red blood cell transfusions. Young patients with adequate myocardial reserve tolerated hematocrit levels of as low as 20% without the necessity of transfusions. The indication of surgical intervention for hemostasis followed certain individual variations, according to the surgeon in charge. In general, it was indicated when the blood loss was higher that 500 mL within the first postoperative hour, greater than 300 mL for two consecutive hours or greater than 1 liter within the first eight postoperative hours.
The variables studied are listed in Appendix I. The preoperative variables were collected on the eve of the operation by a direct interview of the patient or direct consultation of the patient's records. The intraoperative variables were collected by researchers soon after the surgery by studying the description of the surgery, registers of the anesthetist and perfusion records, if applicable. In the postoperative period, the variables were collected from the vital sign control records and routine laboratory examinations within the first 24 postoperative hours.
Statistic analysis
The categorical variables were expressed as frequencies and percentages and the constant variables as means ± standard deviations. Mediastinal bleeding within 24 hours was studied as a continuous variable. To determine the risk factors for bleeding, the Student t-test for non-paired values and the Pearson correlation test for univariant analysis were employed. The multivariate analysis was performed using multiple linear regressions. They were considered expressive when the p-value was less than 0.05, with a confidence interval of 95%.
RESULTS
The 30-day mortality rate was 5.6% (23 patients). The mean volume of bleeding over 24 hours was 610 ± 500 mL (range from 10 to 4900 mL). Surgical intervention for hemostasis was necessary in 15 (3.7%) patients. The characteristics of these patients including the bleeding volume until the surgical intervention, the length of time until the intervention and the operative findings are shown in Table 2. The origin of the bleeding was not identified by surgical intervention for hemostasis in 9 (60%) patients, and in these cases the cause was attributed to postoperative coagulopathy. The most frequent cause of bleeding in the remaining patients (3 patients - 20%) was laceration of the intercostal vessel linked to the passage of wires during closure of the sternum.
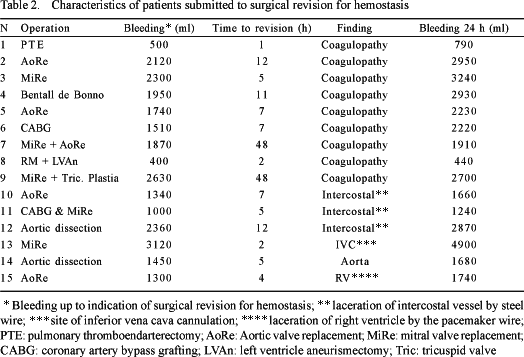
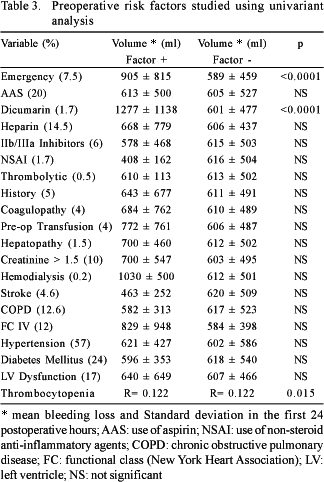
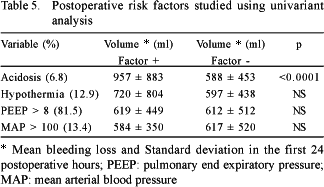
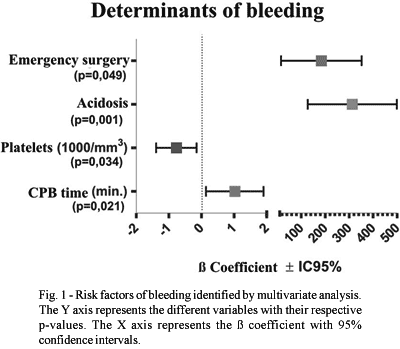
The risk factors for bleeding are shown in Tables 3 to 5. The factors associated to the greatest blood loss within the first 24 postoperative hours were, according to univariant analysis: emergency operations, the use of dicumarinic anticoagulants, preoperative thrombocytopenia, the use of cardiopulmonary bypass, high doses of heparin, prolonged CPB time, CPB temperature, surgery of the aorta and metabolic acidosis in the postoperative period. Reoperations, ingestion of aspirin less than five days before surgery and the lack of intraoperative infusion of antifibrinolytic agents did not influence the postoperative bleeding volume.
Figure 1 shows the predictive bleeding factors identified by multivariate analysis. Emergency surgery (p=0.049), postoperative metabolic acidosis (p=0.001), preoperative thrombocytopenia (p=0.034) and prolonged cardiopulmonary bypass time (0.02l) were recognized as significant factors.
COMMENTS
Blood loss resulting from bleeding and the attempt to correct hemostasis disorders are responsible for increases in transfusional rates. Despite of improvements in the methods of donator selection, blood and blood component transfusion is associated with several complications, such as viral infections, induction of immunologically caused transfusional reactions and suppression of the immune system.
Surgical intervention for hemostasis is necessary in those patients with acute or persistent bleeding that can not be explained by coagulation disorders and that does not answer to the correction of specific factors. In our series, 3.7% of the patients needed surgical intervention for hemostasis, a result similar to the prevalence reported by other studies [1,2]. In a little less than half of the patients [1] the source of bleeding was not found and so a coagulopathy was usually stated as the principal cause. The clinical repercussions of surgical intervention for hemostasis are important [1], as it increases by three-fold hospital mortality and increases by four-fold the occurrence of acute renal insufficiency and sepsis.
Mediastinal bleeding in heart surgery is multifactorial. Perioperative bleeding is linked to surgical injury of blood vessels and defects of the hemostatic mechanisms. CPB is considered one of the most important factors for bleeding, according to several studies that discuss this subject. [1,3,12-14] Coagulation disorders are normally linked to exposure of elements in the blood to the CPB circuit. It has been proved that CPB causes a reduction in the levels of coagulation factors, stimulates fibrinolysis, induces thrombocytopenia [14], disseminating intravascular coagulation and platelet dysfunction [15,16]. Also the effects of circulating heparin and protamine must be considered [17]. Our findings confirm this hypothesis, as the use of CPB, its temperature and, specifically, its prolonged use were directly associated to greater bleeding within the first 24 postoperative hours. The preoperative thrombocytopenia was one of the independent risk factors for bleeding, which was aggravated by the CPB.
According to previous studies [18-20], emergency surgeries presented with more bleeding than elective procedures. This fact is attributed to the prior use of aspirin and protein IIb/IIIa inhibitors, which was not confirmed in this study. Aspirin as a risk factor for bleeding is controversial [21,22]. Although we did not evidence more bleeding in patients who had ingested aspirin, the operation was usually postponed for one week, depending on the preoperative clinical conditions. In respect to protein IIb/IIIa inhibitors, the surgeries were scheduled for 12 hours after drug cessation, which could have influenced our results. BIZZAARRI et al. [23] did not identify differences in the postoperative bleeding in patients who had taken tirofiban as little as two hours before emergency surgery.
Unfractioned or low molecular weight heparin, at any time in the preoperative period, did not influence the bleeding. KINCAID et al. [24] found greater bleeding in patients who received low molecular weight heparin less than 12 hours before surgery; a fact that must be related to the anti-Xa effect, with a peak action at 12 hours in 30% of the cases, in spite of having a half-life of from 5 to 6 hours.
Both heparin and protamine doses did not altered the volume of bleeding in the postoperative period. The control of the activated coagulation time is the safest strategy to identify the necessity of additional neutralization of circulating heparin. The empirical infusion of extra doses of protamine in patients with bleeding can increase the blood loss [3]. However, additional doses of 30% of total protamine dose in the first hours of the postoperative period demonstrated to be efficient in the control of the rebound effect of heparin, reducing bleeding and the necessity of transfusions [25].
Reoperations and associated procedures are normally associated with greater bleeding. The use of antifibrinolytic agents has proved efficient in the reduction of bleeding and the necessity of transfusion in randomized prospective studies [10,11]. In this series, significant differences were not observed, maybe because the antifibrinolytic drugs neutralized the greater risk of bleeding in patients submitted to reoperations and associated procedures.
In respect to the postoperative period, metabolic acidosis was the only independent risk factor associated to bleeding. Generally this was caused by low cardiac output either owing to cardiogenic shock or hypovolemia. Hypothermia has been implicated as a risk factor in other studies, [3,14], but it was not associated to the volume of blood loss in our series.
This study presents with some limitations. The number of events resulting in death or surgical intervention for hemostasis was low and so analysis of the association of bleeding and these two variables was not possible. Transfusional requirements were not studied.
This work confirms the concepts that patients submitted in emergency surgery and those with thrombocytopenia require optimization of the preoperative clinical conditions. The use of cardiopulmonary bypasses must be minimized, principally in respect of the time of use. Metabolic acidosis in postoperative period must be aggressively corrected, aiming at identifying its main cause.
BIBLIOGRAPHIC REFERENCES
1. Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg 1996;111:1037-46.
[ Medline ]
2. Unsworth-White MJ, Herriot A, Valencia O, Poloniecki J, Smith EE, Murday AJ et al. Resternotomy for bleeding after cardiac operation: a marker for increased morbidity and mortality. Ann Thorac Surg 1995;59:664-7.
[ Medline ]
3. Despotis GJ, Filos KS, Zoys TN, Hogue Jr. CW, Spitznagel E, Lappas DG. Factors associated with excessive postoperative blood loss and hemostatic transfusion requirements: a multivariate analysis in cardiac surgical patients. Anesth Analg 1996;82:13-21.
[ Medline ]
4. Helm RE, Rosengart TK, Gomez M, Klemperer JD, DeBois WJ, Velasco F et al. Comprehensive multimodality blood conservation: 100 consecutive CABG operations without transfusion. Ann Thorac Surg 1998;65:125-36.
[ Medline ]
5. Sakert T, Gil W, Rosenberg I, Carpellotti D, Boss K, Williams T et al. Cell saver efficacy for routine coronary artery bypass surgery. Perfusion 1996;11:71-7.
[ Medline ]
6. Schaff HV, Hauer J, Gardner TJ, Donahoo JS, Watkins Jr L, Gott VL et al. Routine use of autotransfusion following cardiac surgery: experience in 700 patients. Ann Thorac Surg 1979;27:493-9.
[ Medline ]
7. Aldea GS, Zhang X, Memmolo CA, Shapira OM, Treanor PR, Kupferschmid JP et al. Enhanced blood conservation in primary coronary artery bypass surgery using heparin-bonded circuits with lower anticoagulation. J Card Surg 1996;11:85-95.
[ Medline ]
8. van de Watering LM, Hermans J, Houbiers JG, van den Broek PJ, Bouter H, Boer F et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation 1998;97:562-8.
[ Medline ]
9. Lemmer JH Jr, Stanford W, Bonney SL, Breen JF, Chomka EV, Eldredge WJ et al. Aprotinin for coronary bypass operations: efficacy, safety, and influence on early saphenous vein graft patency. A multicenter, randomized, double-blind, placebo-controlled study. J Thorac Cardiovasc Surg 1994;107:543-53.
[ Medline ]
10. Munoz JJ, Birkmeyer NJ, Birkmeyer JD, O'Connor GT, Dacey LJ. Is epsilon-aminocaproic acid as effective as aprotinin in reducing bleeding with cardiac surgery? A meta-analysis. Circulation 1999;99:81-9.
[ Medline ]
11. Gonçalves FD, Novaes FR, Maia MA. Influência do ácido tranexâmico no sangramento pós-operatório de cirurgias cardíacas com circulação extracorpórea. Rev Bras Cir Cardiovasc 2002;17:331-8.
[ SciELO ]
12. Ascione R, Williams S, Lloyd CT, Sundaramoorthi T, Pitsis AA, Angelini GD. Reduced postoperative blood loss and transfusion requirement after beating-heart coronary operations: a prospective randomized study. J Thorac Cardiovasc Surg 2001;121:689-96.
[ Medline ]
13. Scott BH, Seifert FC, Glass PS, Grimson R. Blood use in patients undergoing coronary artery bypass surgery: impact of cardiopulmonary bypass pump, hematocrit, gender, age and body weight. Anesth Analg 2003;97:958-63.
[ Medline ]
14. Khuri SF, Wolfe JA, Josa M, Axford TC, Szymanski I, Assousa S et al. Hematologic changes during and after cardiopulmonary bypass and their relationship to the bleeding time and nonsurgical blood loss. J Thorac Cardiovasc Surg 1992;104:94-107.
[ Medline ]
15. Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood 1990;76:1680-97.
[ Medline ]
16. Czer LS. Mediastinal bleeding after cardiac surgery: etiologies, diagnostic considerations and blood conservation methods. J Cardiothorac Anesth 1989;3:760-75.
[ Medline ]
17. Milas BL, Jobes DR, Gorman RC. Management of bleeding and coagulopathy after heart surgery. Semin Thorac Cardiovasc Surg 2000;12:326-36.
[ Medline ]
18. Grubitzsch H, Wollert HG, Eckel L. Emergency coronary artery bypass grafting: does excessive preoperative anticoagulation increase bleeding complications and transfusion requirements? Cardiovasc Surg 2001;9:510-6.
[ Medline ]
19. Ferraris VA, Ferraris SP, Lough FC, Berry WR. Preoperative aspirin ingestion increases operative blood loss after coronary artery bypass grafting. Ann Thorac Surg 1988;45:71-4.
[ Medline ]
20. Gammie JS, Zenati M, Kormos RL, Hattler BG, Wei LM, Pellegrini RV et al. Abciximab and excessive bleeding in patients undergoing emergency cardiac operations. Ann Thorac Surg 1998;65:465-9.
[ Medline ]
21. Vuylsteke A, Oduro A, Cardan E, Latimer RD. Effect of aspirin in coronary artery bypass grafting. J Cardiothorac Vasc Anesth 1997;11:831-4.
[ Medline ]
22. Bashein G, Nessly ML, Rice AL, Counts RB, Misbach GA. Preoperative aspirin therapy and reoperation for bleeding after coronary artery bypass surgery. Arch Intern Med 1991;151:89-93.
[ Medline ]
23. Bizzarri F, Scolletta S, Tucci E, Lucidi M, Davoli G, Toscano Tet al. Perioperative use of tirofiban hydrochloride (Aggrastat) does not increase surgical bleeding after emergency or urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg 2001;122:1181-5.
[ Medline ]
24. Kincaid EH, Monroe ML, Saliba DL, Kon ND, Byerly WG, Reichert MG. Effects of preoperative enoxaparin versus unfractioned heparin on bleeding indices in patients undergoing coronary artery bypass grafting. Ann Thorac Surg 2003;76:124-8.
25. Barroso RC, Mendonça JT, Carvalho MR, Costa RK, Santos JE. Avaliação da protamina na neutralização da heparina após circulação extracorpórea. Rev Bras Cir Cardiovasc 2002;17:54-60.
[ Lilacs ] [ SciELO ]
APPENDIX I
Perioperative variables studied as risk factors for postoperative bleeding in heart surgery
Preoperative variables: emergency surgery, history of bleeding, use of aspirin within five days of surgery, use of anticoagulants within seven days of surgery, use of preoperative heparin, use of protein IIb/IIIa inhibitors, use of non-steroid anti-inflammatory drugs, use of thrombolytic agents, preoperative necessity of blood component transfusion, hepatopathy, chronic renal insufficiency (creatinine greater than 1.5 or the necessity of preoperative hemodialysis), stroke, chronic obstructive pulmonary disease, functional class (New York Heart Association), systemic arterial hypertension, diabetes mellitus, left ventricle ejection fraction, prior hematocrit, platelet count, prothrombin time, international normalization ratio, partial activated thromboplastin time
Intra-operative variables: use of aminocaproic epsilon acid, use of aprotinin, use of cell saver, surgeon, anesthesia time, total dose of heparin and protamine, necessity of CPB, CPB time, aortic clamping time, necessity of total circulatory arrest, lowest temperature during CPB, pre- and post-CPB activated coagulation times, type of procedure, number of steel wires, number of drains.
Postoperative variables (first 24 hours): hypothermia (axillary temperature less than 35 ºC), presence of metabolic acidosis (arterial pH less than 7.2 and serum bicarbonate less than 15), presence of hypertensive peak (arterial blood pressure greater than 100 mmHg), pulmonary end expiratory pressure less than 8.


 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license