INTRODUCTION
COX [1] in 1991 proposed a technique for surgical treatment of primary atrial fibrillation, based on multiple incisions and sutures in the right and left atria giving satisfactory results in the recover of atrial synchronism.
JATENE et al. [2] in 1992 confirmed the benefits of the `maze' technique in patients with rheumatic valvar disease and GREGORI JR. et al. [3] in 1993 reduced part of the complexity of this operation demonstrating its feasibility without the necessity of cryoablation.
Aiming at optimizing the reproducibility of the operation and reducing its risk in relation to the multiple atrial incisions and with the increased adherences in reoperations, alternative surgeries were proposed by JAZBIK et al. [4], BENUSSI et al. [5], KOTTKAMP et al. [6] and KALIL et al. [7], limiting the treatment to the left atrium, as in the original proposals by COUMEL et al. [8] and WILLIAMS et al. [9].
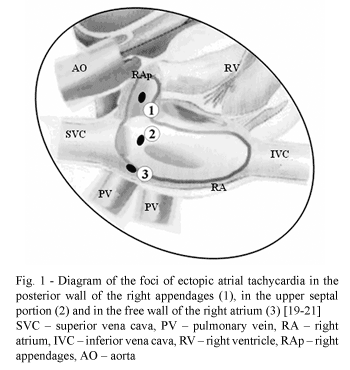
However, the existence of arrhythmogenic foci located in the right appendages, in the upper portion of the interatrial septum and the free wall of the right atrium have also been demonstrated [10,11].
A special contribution was seen with the studies of FRAME et al. [12] in 1987, who accurately described the electrophysiologic anatomy of the four main circuits of reentry to the right atrium, which are: two at the outlets of the upper and lower vena cavas to the right atrium, one medium-atrial encircling the medium portion of the free wall of the atria and the interatrial septum and one para-tricuspid circuit, near to the right atrium junction with the tricuspid valvar annulus (Figure 2).
This study describes the initial results obtained from an optimized biatrial approach in patients with rheumatic valvar diseases, based on electrophysiologic concepts, which had not been considered previously in the surgical procedures for the treatment of chronic atrial fibrillation.
METHOD
With the study project approved by the Ethics Committee of the Sao Francisco Cardiovascular Foundation of Assis/ServCor, eight symptomatic patients (NYHA II/III) with mitral valve dysfunction of a rheumatic etiology and symptomatic tricuspid valvar insufficiency in one case evolving to chronic arterial fibrillation over a period of at least one year were operated on. The patients were submitted to general anesthesia, longitudinal sternotomy and surgical treatment of the valvar dysfunctions and atrial fibrillation.
Six (75%) patients were female and ages ranged from 21 to 67 years old (mean 35.1 years) - Table 1.
Cardiopulmonary bypass with hypothermia (30 - 32 ºC) were employed with total venous drainage by means of cannulae introduced to the superior and inferior vena cavas through areas delimited by purse-string sutures in the right atrium with the suture of the superior vena cava positioned about 5 mm from the base of the right appendages (Figure 1).
The cannula for systemic arterial perfusion was introduced in the distal portion of the ascending aorta and the cannula for cardioplegic arterial perfusion was introduced in its proximal portion.
Myocardial protection was achieved with perfusion of continuous, hypothermiac (30 -32 ºC), hyperkalaemic (25 mEq/L) sanguineous coronary perfusion at 60 to 90 mmHg in a circuit independent of the systemic arterial perfusion (Model Comex Ind. Com. Ltda, BH - MG).
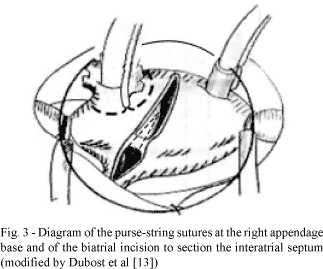
To access the mitral valve, the atrial ostia of the pulmonary veins and left appendages, as well as a sectioning of the, septal and right atrial interatrial conduction zones, a single oblique incision was employed as proposed by DUBOST et al. [13], starting 5 mm above the right atrioventricular groove and stretching 15 mm around the anterior portion of the right superior pulmonary vein, with sectioning of the interatrial septum up to 10 mm above the annulus of the tricuspid valve (Figure 3).
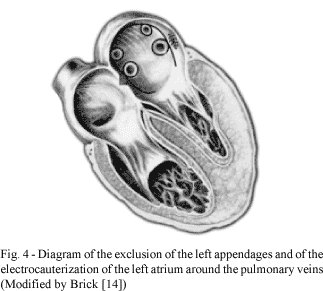
Compartmentalization of the left atrium was achieved using running sutures using 3-0 polypropylene thread in the first two cases and using an electric scalpel in the others, attempting to isolate the ostia of the pulmonary veins from the left appendages, similar to the system used by WILLIAMS et al. [9] and modified by BRICK [14] (Figure 4).
Electrocauterization was performed with a sufficient intensity to pass through the endocardium, exposing the subjacent atrial muscles but avoiding perforation of the chamber itself. Another concern was the cauterization of the atrium, leaving a border of at least 5 mm around the ostia of the pulmonary veins.
The left appendage was excluded by a running suture from its base, in the communication ostium with the left atrium.
In the patient with associated tricuspid insufficiency, a De Veja-type annuloplasty was performed [15], with plicature a ¼ way around the tricuspid annulus (from 12 o'clock to 3 o'clock).
For the reconstruction of the interatrial septum of the right superior pulmonary vein and the right atrial wall, continuous sutures on a single plane were used with a 3-0 polypropylene thread.
The right appendage was excluded by simply closure using purse-string sutures, after the removal of the superior vena cava cannula.
RESULTS
A regular atrial heart rhythm was achieved in all the patients, with hemodynamic stabilization at the end of the cardiopulmonary bypass.
The perfusion time ranged from 64 to 133 minutes with a mean of 107.5 minutes and the aortic clamping varied from 40 to 105 minutes with an average of 76.7 minutes. All the patients were released from hospital with the regular atrial heart rhythm maintained.
During hospitalization, two patients presented with atrial fibrillation which was reverted using digital and amiodarone.
All the patients were released in stable clinical conditions, with a regular rhythm and with the presence of a morphologically varying P-wave. No patients required the permanent implantation of a pacemaker. In the six-month postoperative follow up, two patients returned to an atrial fibrillation rhythm, with the others (75%) continued with regular atrial rhythms, with preservation of the atrial contraction and absence of thrombosis as evidenced by control echocardiograms.
COMMENTS
The historical basis of modern surgical treatment of atrial fibrillation initiated with the demonstration that the presence of left atrial striated musculature extended to the pulmonary veins, reported in 1836 by RAUSCHEL [16] and in 1869 by ELISCHER [17]. In 1914 ROTHBERGER & WINTERBERG [18] postulated electrophysiologic concepts considering that the same single ectopic focus could determine the total atrial fibrillation.
LEWIS [19] and LEWIS et al. [20], in 1920, postulated the importance of the circular movement of the reentry circuits and GARREY [21], in 1924, studied the atrial segmentation to block the propagation of the abnormal stimuli and impede total atrial fibrillation, a concept adopted in the surgical approach of the Cox operation.
However, it was COUMEL et al. [8], in 1973, who performed the first surgery for the treatment of the ectopic focus of arrhythmia located in the left atrial, causing the subsequent development of techniques to isolate the left atrial, to treat the atrial fibrillation.
With the right atrium and the interatrial septum, some studies demonstrated the possibility of treating tachyarrhythmias with the ablation of the arrhythmogenic foci located in the walls of the right appendage, in the upper portion of the interatrial septum and/or the free atrial wall [10,11,22] and the studies of FRAME et al. [12,23] defined the main circuits responsible for sustained tachyarrhythmias. Considering this surgical anatomy of arrhythmogenesis of the right atrium and remembering that ample surgical exposure facilitates the septation of the left atrium, it is thought that the biatrial incision, proposed by DUBOST et al. [13] for the surgery of the mitral valve, would also facilitate the electroablation and electro-septation in the left atrium at the same time in which it would block arrhythmogenic circuits in the free wall of the right atrium and in the upper septal portion.
The relative complexity and the potential increase of morbidity of the `maze' surgery [24-26] has motivated the procedure to be restricted to the left atrium and only using an ultrasound scalpel [27], or by applying radiofrequency [28], reducing sectioning and the sutures necessary in the atrial walls, keeping, however, the success of the conversion to regular atrial rhythm in 70 to 80% of the patients.
In this study, we opted for a simple electric scalpel (electrocauterization or diathermic scalpel), as defined by BATH [29] and also based on satisfactory results obtained by BRICK (personal communication). Special care was taken with the atrial electrocauterization, respecting a border of a minimum of 5 mm around the ostia of the pulmonary veins to prevent stenosis [30]. The technique utilized in this work optimized the atrial fibrillation surgery, substituting the resection of the right and left appendages for purse-string suturing of the entry of the cannula in the superior vena cava in the right atrium, and by the closing suture of the left appendage neck through the left intra-atrial membrane, respectively. Also the ample internal exposure of the left atrium was facilitated by the technique of DUBOST et al. [13], reducing the length of the sutures in the right atrium. From the electrophysiologic point of view, the single incision in the right atrium excluded one of the circuits of reentry of the stimuli in the atrial wall and also the septal channels. As the circuits near to the ostia of the superior and inferior vena cavas were acknowledged by FRAME et al. [12] as being incapable of generating tachyarrhythmias, their ablation can be easily achieved by applying radiofrequency or epimyocardial diathermia in cases of persistent atrial fibrillation at the end of the operation. The last option, in refractory cases, may be, the interruption of the para-tricuspid circuit of FRAME et al. [12], that can be blocked by intracardiac electrocauterization and sectioning the interatrial septum to the tricuspid valve or externally, prolonging atriotomy of the free wall of the right atrium to the atrioventricular groove, taking care not to injure the right coronary artery. The block of this circuit, however, might make the functioning of the atrioventricular node difficult and cause the necessity of the definitive implantation of a pacemaker.
In recent studies, CHIAPPINI et al. [31] and COX et al. [32] proved the convenience and advantages of procedures with lesser morbidity and less surgical complexity in the general context of treatment for atrial fibrillation, which stresses the potential benefits of the approach defined in this study.
In a general analysis of this initial study, we conclude that the technique employed and the electrophysiologic basis considered, simplified the surgery, practically reducing the operation to a single atrial incision and determining the different stages to block the potentially arrhythmogenic circuits, enabling the treatment of the atrial fibrillation and the correction of the mitral and tricuspid valve lesions without additional morbidity. The same criteria of electrophysiologic blocks facilitate the determining of endovascular treatment of atrial fibrillation, when direct intervention of the mitral and tricuspid valves is not necessary.
BIBLIOGRAPHIC REFERENCES
1. Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 1991;101:584-92.
[ Medline ]
2. Jatene AD, Sosa E, Tarasoutchi F, Jatene MB, Pomerantzeff PMA. Tratamento cirúrgico da fibrilação atrial. Procedimento do "labirinto": experiência inicial. Rev Bras Cir Cardiovasc 1992;7:107-11.
[ Lilacs ]
3. Gregori Jr F, Cordeiro C, Goulart M, Couto N, Rosa V, Silva SS et al. Técnica de Cox sem crioablação para tratamento cirúrgico da fibrilação atrial. Rev Bras Cir Cardiovasc 1993;8:220-4.
[ Lilacs ]
4. Jazbik JC, Coutinho JH, Amar MR. Tratamento cirúrgico da fibrilação atrial em pacientes com insuficiência mitral: proposta inicial de uma nova abordagem cirúrgica. Rev SOCERJ1993;6:142-5.
5. Benussi S, Pappone C, Nascimbene S, Oreto G, Caldarola A, Stefano PL et al. A simple way to treat chronic atrial fibrillation during mitral valve surgery: the epicardial radiofrequency approach. Eur J Cardiothorac Surg 2000;17:524-9.
[ Medline ]
6. Kottkamp H, Hindricks G, Hammel D, Autschbach R, Mergenthaler J, Borggrefe M et al. Intraoperative radiofrequency ablation of chronic atrial fibrillation: a left atrial curative approach by elimination of anatomic "anchor" reentrant circuits. J Cardiovasc Electrophysiol 1999;10:772-80.
[ Medline ]
7. Kalil RAK, Lima GG, Abrahão R, Sturmer ML, Albrecht A, Moreno P et al. Técnica cirúrgica simplificada pode ser eficaz no tratamento da fibrilação atrial crônica secundária a lesão valvar mitral? Rev Bras Cir Cardiovasc 2000;15:129-35.
[ Lilacs ] [ SciELO ]
8. Coumel P, Aigueperse J, Perrault MA, Fantoni A, Scama R, Bouvrain Y. Reperrage et tentative d' exerese chirurgicale d'un foyer ectopique auriculaire gauche avec tachycardie rebelle: evolution favorable. Ann Cardiol Angeiol 1973;22:189-99.
9. Williams JM, Ungerleider RM, Lofland GK, Cox JL. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg 1980;80:373-80.
[ Medline ]
10. Wyndham CR, Arnsdorf MF, Levitsky S, Smith TC, Dhingra RC, Denes P et al. Successful surgical excision of focal paroxysmal atrial tachycardia. Observations in vivo and in vitro. Circulation 1980;62:1365-72.
11. Graffigna A, Vigano M, Pagani F, Salerno G. Surgical treatment for ectopic atrial tachycardia. Ann Thorac Surg 1992;54:338-43.
[ Medline ]
12. Frame LH, Page RL, Boyden PA, Fenoglio Jr. JJ, Hoffman BF. Circus movement in the canine atrium around the tricuspid ring during experimental atrial flutter and during reentry in vitro. Circulation 1987;76:1155-75.
[ Medline ]
13. Dubost C, Guilmet D, de Parades B, Pedeferri G. New technique of opening of the left auricle in open-heart surgery: the transseptal bi-auricular approach. Presse Med 1966;74:1607-8.
14. Brick AV. Tratamento intraoperatório da fibrilação atrial crônica com ultra-som [Tese de Mestrado]. Belo Horizonte: Fundação Cardiovascular São Francisco de Assis, 2000.
15. Gomes OM, Pitchon M. Anuloplastia tricúspide parcial (1/4). Coração 1986;1:3.
16. Rauschel F. De arteriarum et venarum structura, Breslau, 1836. In: Burch GE, Romney RB. Functional anatomy and "throttle valve" action of the pulmonary veins. Am Heart J 1954;47:58-68.
17. Elischer J. Ueber quergestreifte muskeln der ins herz mündenden venen des menschen, 1869. In: Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins: an anatomic study of human hearts. Circulation 1966;34:412-22.
18. Rothberger CJ, Winterberg H. Uber vorhofflimmern und vorhofflattern, 1914. In: Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:402-5.
[ Medline ]
19. Lewis T. Observations upon flutter and fibrillation. Part IV. Impure flutter: theory of circus movement. Heart 1920;7:293-331.
20. Lewis T, Feil HS, Stroud WD. Observations upon flutter and fibrillation. Part II - The nature of auricular flutter. Heart 1920;7:191. In: Frame LH, Page RL, Boyden PA, Fenoglio JJ, Hoffman BF. Circus movement in the canine atrium around the tricuspid ring during experimental atrial flutter and during reentry in vitro. Circulation 1987; 76:1155-75.
[ Medline ]
21. Garrey WE. Auricular fibrillation. Physiol Rev 1924;4:215-50.
22. Josephson ME, Spear JF, Harken AH, Horowitz LN, Dorio RJ. Surgical excision of automatic atrial tachycardia: anatomic and eletrophysiologic correlates. Am Heart J 1982;104:1076-85.
[ Medline ]
23. Frame LH, Page RL, Boyden PA, Hoffman BF. A right atrial incision that stabilized reentry around the tricuspid ring in dogs. Circulation 1983;68(suppl III):III-361.
24. McCarthy PM, Castle LW, Maloney JD, Trohman RG, Simmons TW, White RD et al. Initial experience with the maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg 1993;105:1077-87.
[ Medline ]
25. Jatene MB, Sosa E, Jatene FB, Tarasoutchi F, Monteiro AC, Salerno PR et al. Evolução tardia da operação de Cox para fibrilação atrial em valvopatia mitral. Rev Bras Cir Cardiovasc 1995;10:18-24.
[ Lilacs ]
26. Kawaguchi AT, Kosakai Y, Sasako Y, Eishi K, Nakano K, Kawashima Y. Risks and benefits of combined maze procedure for atrial fibrillation associated with organic heart disease. J Am Coll Cardiol 1996;28:985-90.
[ Medline ]
27. Brick AV, Seixas T, Peres A, Vieira Jr. JJ, Mattos JV, Mesquita A et al. Reversão da fibrilação atrial crônica pela técnica do labirinto com aplicação de radiofreqüência e ultra-som transoperatórios. Rev Bras Cir Cardiovasc 1999;14:290-7.
[ Lilacs ] [ SciELO ]
28. Wanderlei Neto J. Tratamento cirúrgico da fibrilação atrial. In: 30º Congresso Nacional de Cirurgia Cardíaca / Simpósio DEPEX-SBCCV. Goiânia: Sociedade Brasileira de Cirurgia Cardiovascular; 2003.
29. Bath PS (Conference discussion. Appendix A) In: Benussi S, Pappone C, Nascimbene S, Oreto G, Caldarola A, Stefano PL et al. A simple way to treat chronic atrial fibrillation during mitral valve surgery: the epicardial radiofrequency approach. Eur J Cardiothoracic Surg 2000;17:524-9.
30. Robbins IM, Colvin EV, Doyle TP, Kemp WE, Loyd JE, McMahon WS et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998;98:1769-75.
[ Medline ]
31. Chiappini B, Martìn-Suàrez S, LoForte A, Arpesella G, Di Bartolomeo R, Marinelli G. Cox/Maze III operation versus radiofrequency ablation for the surgical treatment of atrial fibrillation: a comparative study. Ann Thorac Surg 2004;77:87-92.
[ Medline ]
32. Cox JL. Atrial fibrillation II: rationale for surgical treatment. J Thorac Cardiovasc Surg 2003;126:1693-9.
[ Medline ]


 All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license
All scientific articles published at www.bjcvs.org are licensed under a Creative Commons license